The pyrrolidine alkaloids are natural products chemically derived from pyrrolidine. [1]

The pyrrolidine alkaloids are natural products chemically derived from pyrrolidine. [1]
Alkaloids with partial pyrrolidine structure are usually sub-categorized based on their occurrence and biogenetic origin. Hygrin and cuscohygrin were isolated from the leaves of the coca shrub, [2] while (-)-codonopsinine was isolated from the woodland vine tiger bell. [3]
Among the most important representatives of the pyrrolidine alkaloids are hygrin and cuscohygrin. [2] Another representative is the (-)-codonopsinine. [3] Furthermore, ruspolinone, norruspolinone and norruspoline also belong to this alkaloid group. [4]
Many plants containing cuscohygrin are used in the folk medicine of various peoples as sedatives or narcotics. [5]

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..

Pyridine alkaloids are a class of alkaloids, nitrogen-containing chemical compounds widely found in plants, that contain a pyridine ring. Examples include nicotine and anabasine which are found in plants of the genus Nicotiana including tobacco.

Piperidine alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from piperidine.

Acridone alkaloids are natural products derived from acridone.

Isoquinoline alkaloids are natural products of the group of alkaloids, which are chemically derived from isoquinoline. They form the largest group among the alkaloids.

Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids.
Quinoline alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from quinoline. Some quinoline alkaloids show antiseptic, convulsive or antineoplastic effects.
Erythrina alkaloids, generally containing benzyl-tetrahydroisoquinoline structure, are widely distributed in Erythrina species, a genus of plants which belong to the Fabaceae family in tropical and subtropical regions. The Erythrina alkaloids can be found in several organs of Erythrina trees but are primarily found in their seeds. They display several unique properties, and are the subject of active scientific research relating to their synthesis and bioactivity.

The carbazole alkaloids are natural products of the indole alkaloid type, derived from carbazole.
The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloids and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.
Bisbenzylisoquinoline alkaloids are natural products found primarily in the plant families of the barberry family, the Menispermaceae, the Monimiaceae, and the buttercup family.

Cephalotaxus alkaloids are natural products characterized by pentacyclic structure.

Indolizidine alkaloids are natural products from various alkaloid groups whose structure can be derived from indolizidine.
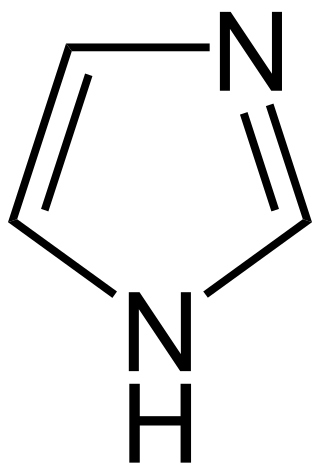
Imidazole alkaloids are a group of alkaloids whose basic structure contains the imidazole ring system.

Corydalis Alkaloids are categorized as natural products of the isoquinoline alkaloid type.

Diterpene alkaloids are natural products of the terpene alkaloid type.

Dendrobium alkaloids are natural products and so-called pseudoalkaloids.

Areca alkaloids are a group of piperidine alkaloids found in the areca nut, the seeds of the areca palm.
Apocynaceae alkaloids are natural products found in the plant family of the dogbane family (Apocynaceae).

Methoxyacrylates are a group of organic compounds primarily used as fungicides. They comprise an acrylic acid ester unit that also includes a methoxy group, making them a type of enol ether.
{{citation}}: CS1 maint: multiple names: authors list (link)