
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (Ea) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius.
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 1884 that the van 't Hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. This equation has a vast and important application in determining the rate of chemical reactions and for calculation of energy of activation. Arrhenius provided a physical justification and interpretation for the formula. Currently, it is best seen as an empirical relationship. It can be used to model the temperature variation of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally-induced processes/reactions. The Eyring equation, developed in 1935, also expresses the relationship between rate and energy.
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by , , or .

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.

The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time.

Exercise physiology is the physiology of physical exercise. It is one of the allied health professions, and involves the study of the acute responses and chronic adaptations to exercise. Exercise physiologists are the highest qualified exercise professionals and utilise education, lifestyle intervention and specific forms of exercise to rehabilitate and manage acute and chronic injuries and conditions.
In biology, a motor unit is made up of a motor neuron and all of the skeletal muscle fibers innervated by the neuron's axon terminals, including the neuromuscular junctions between the neuron and the fibres. Groups of motor units often work together as a motor pool to coordinate the contractions of a single muscle. The concept was proposed by Charles Scott Sherrington.

The diving reflex, also known as the diving response and mammalian diving reflex, is a set of physiological responses to immersion that overrides the basic homeostatic reflexes, and is found in all air-breathing vertebrates studied to date. It optimizes respiration by preferentially distributing oxygen stores to the heart and brain, enabling submersion for an extended time.
Basal metabolic rate (BMR) is the rate of energy expenditure per unit time by endothermic animals at rest. It is reported in energy units per unit time ranging from watt (joule/second) to ml O2/min or joule per hour per kg body mass J/(h·kg). Proper measurement requires a strict set of criteria to be met. These criteria include being in a physically and psychologically undisturbed state and being in a thermally neutral environment while in the post-absorptive state (i.e., not actively digesting food). In bradymetabolic animals, such as fish and reptiles, the equivalent term standard metabolic rate (SMR) applies. It follows the same criteria as BMR, but requires the documentation of the temperature at which the metabolic rate was measured. This makes BMR a variant of standard metabolic rate measurement that excludes the temperature data, a practice that has led to problems in defining "standard" rates of metabolism for many mammals.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.
A temperature coefficient describes the relative change of a physical property that is associated with a given change in temperature. For a property R that changes when the temperature changes by dT, the temperature coefficient α is defined by the following equation:

Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions.
Tachyphylaxis is a medical term describing an acute, sudden decrease in response to a drug after its administration; i.e. a rapid and short-term onset of drug tolerance. It can occur after an initial dose or after a series of small doses. Increasing the dose of the drug may be able to restore the original response.

Coldwater fish can have different meanings in different contexts. In the context of fishkeeping, it refers to fish species that tolerate well the temperatures of a typical indoor aquarium and do not require a heater to remain active; in the context of ecology and fishing, it refers to fish species that prefers to inhabit waterbodies or depth zones with much lower temperatures than the average temperate water.

Shark anatomy differs from that of bony fish in a variety of ways. Variation observed within shark anatomy is a potential result of speciation and habitat variation.
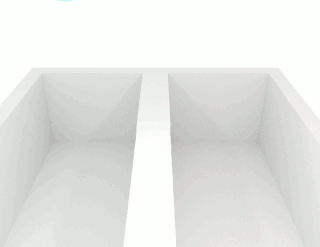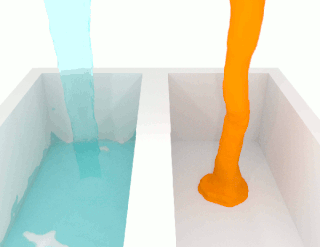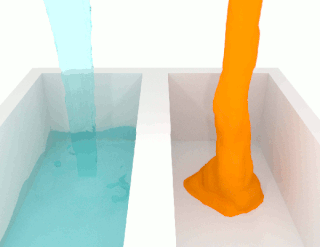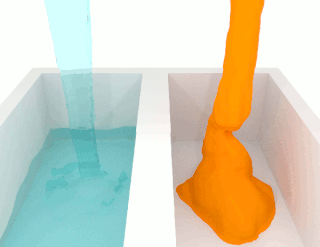
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per square metre, or pascal-seconds.
Ballistic movement can be defined as muscle contractions that exhibit maximum velocities and accelerations over a very short period of time. They exhibit high firing rates, high force production, and very brief contraction times.
In chemical kinetics, the Aquilanti–Mundim deformed Arrhenius model is a generalization of the standard Arrhenius law.
Dynapenia is the loss of muscular strength not caused by neurological or muscular disease that typically is associated with older adults.
Albert Farrell Bennett is an American zoologist, physiologist, evolutionary biologist, author, and academic. He is Dean Emeritus of the School of Biological Sciences at University of California, Irvine.













