Related Research Articles

Salicylic acid is an organic compound with the formula HOC6H4COOH. A colorless (or, white), bitter-tasting solid, it is a precursor to and a metabolite of acetylsalicylic acid (aspirin). It is a plant hormone, and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen. The name is from Latin salix for willow tree, from which it was initially identified and derived. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates.

Tannins are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids.

Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure.

Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".

Sodium hexametaphosphate (SHMP) is a salt of composition Na6[(PO3)6]. Sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: NaPO3), of which the hexamer is one, and is usually the compound referred to by this name. Such a mixture is more correctly termed sodium polymetaphosphate. They are white solids that dissolve in water.

Iron gall ink is a purple-black or brown-black ink made from iron salts and tannic acids from vegetable sources. It was the standard ink formulation used in Europe for the 1400-year period between the 5th and 19th centuries, remained in widespread use well into the 20th century, and is still sold today.

Tannic acid is a specific form of tannin, a type of polyphenol. Its weak acidity (pKa around 6) is due to the numerous phenol groups in the structure. The chemical formula for commercial tannic acid is often given as C76H52O46, which corresponds with decagalloyl glucose, but in fact it is a mixture of polygalloyl glucoses or polygalloyl quinic acid esters with the number of galloyl moieties per molecule ranging from 2 up to 12 depending on the plant source used to extract the tannic acid. Commercial tannic acid is usually extracted from any of the following plant parts: Tara pods (Caesalpinia spinosa), gallnuts from Rhus semialata or Quercus infectoria or Sicilian sumac leaves (Rhus coriaria).

Quercitron is a yellow natural dye obtained from the bark of the Eastern Black Oak, a forest tree indigenous in North America. It was formerly called Dutch pink, English pink, or Italian pink.

Gerardus Johannes Mulder or Gerrit Jan Mulder was a Dutch organic and analytical chemist.
Paraldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in ethanol. Paraldehyde slowly oxidizes in air, turning brown and producing an odour of acetic acid. It attacks most plastics and rubbers and should be kept in glass bottles.

Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula C9H14O or ( 2C=CH)2C=O.

Bdellium is a semi-transparent oleo-gum resin extracted from Commiphora wightii plants, and from Commiphora africana trees growing in sub-saharan Africa. According to Pliny the best quality came from Bactria. Other named sources for the resin are India, Pakistan, Arabia, Media, and Babylon.

Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid.
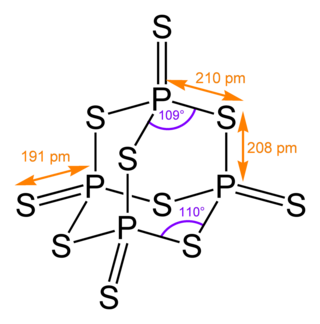
Phosphorus pentasulfide is the inorganic compound with the formula P2S5 (empirical) or P4S10 (molecular). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF.

Benzilic acid is an organic compound with formula C
14H
12O
3 or (C
6H
5)2(HO)C(COOH). It is a white crystalline aromatic acid, soluble in many primary alcohols.
Strontium iodide is an inorganic compound with the chemical formula SrI2. It is a salt of strontium and iodine. It forms a hexahydrate SrI2·6H2O. It is an ionic, water-soluble, and deliquescent compound that can be used in medicine as a substitute for potassium iodide. It is also used as a scintillation gamma radiation detector, typically doped with europium, due to its optical clarity, relatively high density, high effective atomic number (Z=48), and high scintillation light yield. In recent years, europium-doped strontium iodide (SrI2:Eu2+) has emerged as a promising scintillation material for gamma-ray spectroscopy with extremely high light yield and proportional response, exceeding that of the widely used high performance commercial scintillator LaBr3:Ce3+. Large diameter SrI2 crystals can be grown reliably using vertical Bridgman technique and are being commercialized by several companies.

4,4′-Bipyridine (abbreviated to 4,4′-bipy or 4,4′-bpy) is an organic compound with the formula (C5H4N)2. It is one of several isomers of bipyridine. It is a colorless solid that is soluble in organic solvents. is mainly used as a precursor to N,N′-dimethyl-4,4′-bipyridinium [(C5H4NCH3)2]2+, known as paraquat.

Phlobaphenes are reddish, alcohol-soluble and water-insoluble phenolic substances. They can be extracted from plants, or be the result from treatment of tannin extracts with mineral acids. The name phlobaphen come from the Greek roots φλoιὀς (phloios) meaning bark and βαφή (baphe) meaning dye.

Quercus infectoria or the Aleppo oak is a species of oak well known for producing galls that have been traditionally used for centuries in Asia medicinally while also used in softening leather and in making black dye and ink.

Taraxacin is a guaianolide with the molecular formula C15H14O3 which has been isolated from the plant Taraxacum officinale. Taraxacin has a bitter taste. Taraxacin has diuretic properties.
References
- ↑ L'Energie Homo-Hydrogne By Patricia Le Roux (French)
- ↑ Quercus on www.henriettesherbal.com
- ↑ Quercitannic on everything2.com
- ↑ Traité de chimie, Volume 2. Jöns Jakob Berzelius (friherre) and Olof Gustaf Öngren, A. Wahlen et Cie., 1838
- ↑ A dictionary of chemistry, Volume 5, by Henry Watts, 1865
- ↑ Über die Gerbsäure der Eichenrinde. C. Etti, Monatshefte für Chemie, Volume 1, Number 1, 262-278, 1880 doi : 10.1007/BF01517069 (German)
- ↑ Zur Geschichte der Eichenrindegerbsäuren. C. Etti, Monatshefte für Chemie, Volume 4, Number 1, 512-530, 1883 doi : 10.1007/BF01517990 (German)
- ↑ The nature of tea infusions. Henry L. Smith, The Lancet, Volume 181, Issue 4673, 22 March 1913, Page 846, doi : 10.1016/S0140-6736(01)03766-7
- ↑ What are the historic and contemporary ethnobotanical uses of native Rhode Island wetlands plants? by Courtney Reckford, 1997
- ↑ A manual of organic materia medica and pharmacognosy - An introduction to the study of the vegetable Kingdom and the vegetable and animal drugs (Sayre's Materia Medica) fourth edition, by Lucius E Sayre
- ↑ Government standards for spices. Journal of the American Pharmaceutical Association, Volume 12, Issue 12, pages 1091–1094, December 1923, doi : 10.1002/jps.3080121212