Related Research Articles

Flow cytometry (FC) is a technique used to detect and measure physical and chemical characteristics of a population of cells or particles.

Immunofluorescence(IF) is a light microscopy-based technique that allows detection and localization of a wide variety of target biomolecules within a cell or tissue at a quantitative level. The technique utilizes the binding specificity of antibodies and antigens. The specific region an antibody recognizes on an antigen is called an epitope. Several antibodies can recognize the same epitope but differ in their binding affinity. The antibody with the higher affinity for a specific epitope will surpass antibodies with a lower affinity for the same epitope.
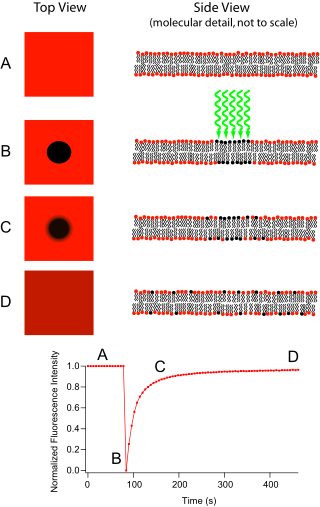
Fluorescence recovery after photobleaching (FRAP) is a method for determining the kinetics of diffusion through tissue or cells. It is capable of quantifying the two-dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes, or to examine single cells. This technique is very useful in biological studies of cell membrane diffusion and protein binding. In addition, surface deposition of a fluorescing phospholipid bilayer allows the characterization of hydrophilic surfaces in terms of surface structure and free energy.

Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance.
A total internal reflection fluorescence microscope (TIRFM) is a type of microscope with which a thin region of a specimen, usually less than 200 nanometers can be observed.

A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image.

Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser scanning confocal microscopy (LSCM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation. Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures within an object. This technique is used extensively in the scientific and industrial communities and typical applications are in life sciences, semiconductor inspection and materials science.
Riken is a national scientific research institute in Japan. Founded in 1917, it now has about 3,000 scientists on seven campuses across Japan, including the main site at Wakō, Saitama Prefecture, on the outskirts of Tokyo. Riken is a Designated National Research and Development Institute, and was formerly an Independent Administrative Institution.

Two-photon excitation microscopy is a fluorescence imaging technique that is particularly well-suited to image scattering living tissue of up to about one millimeter in thickness. Unlike traditional fluorescence microscopy, where the excitation wavelength is shorter than the emission wavelength, two-photon excitation requires simultaneous excitation by two photons with longer wavelength than the emitted light. The laser is focused onto a specific location in the tissue and scanned across the sample to sequentially produce the image. Due to the non-linearity of two-photon excitation, mainly fluorophores in the micrometer-sized focus of the laser beam are excited, which results in the spatial resolution of the image. This contrasts with confocal microscopy, where the spatial resolution is produced by the interaction of excitation focus and the confined detection with a pinhole.

ICFO – The Institute of Photonic Sciences is a research center devoted to the science and technology of light. Located in Castelldefels, ICFO was created in 2002 by the Government of Catalonia and the Technical University of Catalonia.
An X-ray microscope uses electromagnetic radiation in the soft X-ray band to produce images of very small objects.
The Association of Biomolecular Resource Facilities (ABRF) is dedicated to advancing core and research biotechnology laboratories through research, communication, and education. ABRF members include over 2000 scientists representing 340 different core laboratories in 41 countries, including those in industry, government, academic and research institutions.

The Institute of Medical Science is an ancillary establishment of Tokyo University. It succeeded the Institute of Infectious Diseases established in 1892 and is the foremost institute for medical and bioscience research in Japan.

Oxford Instruments Andor Ltd is a global developer and manufacturer of high-performance scientific cameras, microscopy systems and spectrographs for academic, government, and industrial applications. Founded in 1989, the company's products play a central role in the advancement of research in the fields of life sciences, physical sciences, and industrial applications. Andor was purchased for £176 million in December 2013 by Oxford Instruments. The company is based in Belfast, Northern Ireland and now employs over 400 staff across the group at its offices in Belfast, Japan, China, Switzerland and the US.
Fluorescence cross-correlation spectroscopy (FCCS) is a spectroscopic technique that examines the interactions of fluorescent particles of different colours as they randomly diffuse through a microscopic detection volume over time, under steady conditions.

The California Institute for Quantitative Biosciences (QB3) is a nonprofit research and technology commercialization institute affiliated with three University of California campuses in the San Francisco Bay Area: Berkeley, San Francisco, and Santa Cruz. QB3's domain is the quantitative biosciences: areas of biology in which advances are chiefly made by scientists applying techniques from physics, chemistry, engineering, and computer science.
Endomicroscopy is a technique for obtaining histology-like images from inside the human body in real-time, a process known as ‘optical biopsy’. It generally refers to fluorescence confocal microscopy, although multi-photon microscopy and optical coherence tomography have also been adapted for endoscopic use. Commercially available clinical and pre-clinical endomicroscopes can achieve a resolution on the order of a micrometre, have a field-of-view of several hundred µm, and are compatible with fluorophores which are excitable using 488 nm laser light. The main clinical applications are currently in imaging of the tumour margins of the brain and gastro-intestinal tract, particularly for the diagnosis and characterisation of Barrett’s Esophagus, pancreatic cysts and colorectal lesions. A number of pre-clinical and transnational applications have been developed for endomicroscopy as it enables researchers to perform live animal imaging. Major pre-clinical applications are in gastro-intestinal tract, toumour margin detection, uterine complications, ischaemia, live imaging of cartilage and tendon and organoid imaging.

Live-cell imaging is the study of living cells using time-lapse microscopy. It is used by scientists to obtain a better understanding of biological function through the study of cellular dynamics. Live-cell imaging was pioneered in the first decade of the 21st century. One of the first time-lapse microcinematographic films of cells ever made was made by Julius Ries, showing the fertilization and development of the sea urchin egg. Since then, several microscopy methods have been developed to study living cells in greater detail with less effort. A newer type of imaging using quantum dots have been used, as they are shown to be more stable. The development of holotomographic microscopy has disregarded phototoxicity and other staining-derived disadvantages by implementing digital staining based on cells’ refractive index.
Hiroki R. Ueda is a Japanese professor of biology at the University of Tokyo and the RIKEN Quantitative Biology Center. He is known for his studies on the circadian clock.
Peter John O'Toole is a British biologist who is the Director of the Bioscience Technology Facility and the Head of Imaging and Cytometry at University of York. Since 2023, O'Toole has served as the president of the Royal Microscopical Society.
References
- ↑ "Riken Centers & Labs" . Retrieved 9 November 2015.
- ↑ "About Riken" . Retrieved 9 November 2015.
- ↑ Kaszor, Daniel (November 7, 2014). "Japanese scientists use remarkable new technique to make mice nearly invisible (or at least translucent)". National Post. Retrieved 9 November 2015.
- ↑ "QBiC Overview" . Retrieved 9 November 2015.
- ↑ Hayashi, S; Okada, Y (2015). "Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics". Mol. Biol. Cell. 26 (9): 1743–1751. doi:10.1091/mbc.E14-08-1287. PMC 4436784 . PMID 25717185.
- ↑ Tainaka, K; Kubota, SI; Suyama, TQ; Susaki, EA; Perrin, D; Ukai-Tadenuma, M; Ukai, H; Ueda, HR (2014-11-06). "Whole-Body Imaging with Single-Cell Resolution by Tissue Decolorization". Cell. 159 (4): 911–924. doi: 10.1016/j.cell.2014.10.034 . PMID 25417165.
- ↑ Shimizu, Yoshihiro; Inoue, Akio; Tomari, Yukihide; Suzuki, Tsutomu; Yokogawa, Takashi; Nishikawa, Kazuya; Ueda, Takuya (2001). "Nature Citation". Nature Biotechnology. 19 (8): 751–755. doi:10.1038/90802. PMID 11479568.
- ↑ Shimizu, Y.; Ueda, T. (2010-01-01). "PURE Technology". In Endo, Yaeta; Takai, Kazuyuki; Ueda, Takuya (eds.). PURE Technology - Springer. Methods in Molecular Biology. Vol. 607. Humana Press. pp. 11–21. doi:10.1007/978-1-60327-331-2_2. ISBN 978-1-60327-330-5. PMID 20204844.
- ↑ ""Ray" of light – researchers power LED by connecting it to a fish". www.gizmag.com. Retrieved 2016-06-13.