Gene silencing is the regulation of gene expression in a cell to prevent the expression of a certain gene. Gene silencing can occur during either transcription or translation and is often used in research. In particular, methods used to silence genes are being increasingly used to produce therapeutics to combat cancer and other diseases, such as infectious diseases and neurodegenerative disorders.

Small interfering RNA (siRNA), sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA at first non-coding RNA molecules, typically 20-24 base pairs in length, similar to miRNA, and operating within the RNA interference (RNAi) pathway. It interferes with the expression of specific genes with complementary nucleotide sequences by degrading mRNA after transcription, preventing translation.

Dicer, also known as endoribonuclease Dicer or helicase with RNase motif, is an enzyme that in humans is encoded by the DICER1 gene. Being part of the RNase III family, Dicer cleaves double-stranded RNA (dsRNA) and pre-microRNA (pre-miRNA) into short double-stranded RNA fragments called small interfering RNA and microRNA, respectively. These fragments are approximately 20–25 base pairs long with a two-base overhang on the 3′-end. Dicer facilitates the activation of the RNA-induced silencing complex (RISC), which is essential for RNA interference. RISC has a catalytic component Argonaute, which is an endonuclease capable of degrading messenger RNA (mRNA).

P-glycoprotein 1 also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1) or cluster of differentiation 243 (CD243) is an important protein of the cell membrane that pumps many foreign substances out of cells. More formally, it is an ATP-dependent efflux pump with broad substrate specificity. It exists in animals, fungi, and bacteria, and it likely evolved as a defense mechanism against harmful substances.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Cationic liposomes are spherical structures that contain positively charged lipids. Cationic liposomes can vary in size between 40 nm and 500 nm, and they can either have one lipid bilayer (monolamellar) or multiple lipid bilayers (multilamellar). The positive charge of the phospholipids allows cationic liposomes to form complexes with negatively charged nucleic acids through ionic interactions. Upon interacting with nucleic acids, cationic liposomes form clusters of aggregated vesicles. These interactions allow cationic liposomes to condense and encapsulate various therapeutic and diagnostic agents in their aqueous compartment or in their lipid bilayer. These cationic liposome-nucleic acid complexes are also referred to as lipoplexes. Due to the overall positive charge of cationic liposomes, they interact with negatively charged cell membranes more readily than classic liposomes. This positive charge can also create some issues in vivo, such as binding to plasma proteins in the bloodstream, which leads to opsonization. These issues can be reduced by optimizing the physical and chemical properties of cationic liposomes through their lipid composition. Cationic liposomes are increasingly being researched for use as delivery vectors in gene therapy due to their capability to efficiently transfect cells. A common application for cationic liposomes is cancer drug delivery.
RNA activation (RNAa) is a small RNA-guided and Argonaute (Ago)-dependent gene regulation phenomenon in which promoter-targeted short double-stranded RNAs (dsRNAs) induce target gene expression at the transcriptional/epigenetic level. RNAa was first reported in a 2006 PNAS paper by Li et al. who also coined the term "RNAa" as a contrast to RNA interference (RNAi) to describe such gene activation phenomenon. dsRNAs that trigger RNAa have been termed small activating RNA (saRNA). Since the initial discovery of RNAa in human cells, many other groups have made similar observations in different mammalian species including human, non-human primates, rat and mice, plant and C. elegans, suggesting that RNAa is an evolutionarily conserved mechanism of gene regulation.

Microvesicles are a type of extracellular vesicle (EV) that are released from the cell membrane. In multicellular organisms, microvesicles and other EVs are found both in tissues and in many types of body fluids. Delimited by a phospholipid bilayer, microvesicles can be as small as the smallest EVs or as large as 1000 nm. They are considered to be larger, on average, than intracellularly-generated EVs known as exosomes. Microvesicles play a role in intercellular communication and can transport molecules such as mRNA, miRNA, and proteins between cells.

The sigma-2 receptor (σ2R) is a sigma receptor subtype that has attracted attention due to its involvement in diseases such as cancer and neurological diseases. It is currently under investigation for its potential diagnostic and therapeutic uses.
Small activating RNAs (saRNAs) are small double-stranded RNAs (dsRNAs) that target gene promoters to induce transcriptional gene activation in a process known as RNA activation (RNAa).

Stable nucleic acid lipid particles (SNALPs) are microscopic particles approximately 120 nanometers in diameter, smaller than the wavelengths of visible light. They have been used to deliver siRNAs therapeutically to mammals in vivo. In SNALPs, the siRNA is surrounded by a lipid bilayer containing a mixture of cationic and fusogenic lipids, coated with diffusible polyethylene glycol.
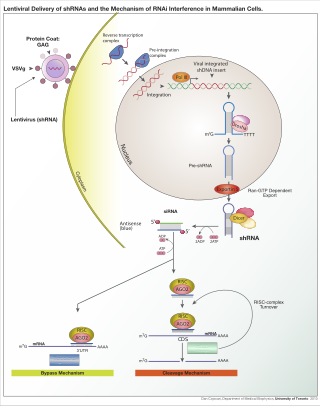
RNA interference (RNAi) is a biological process in which RNA molecules are involved in sequence-specific suppression of gene expression by double-stranded RNA, through translational or transcriptional repression. Historically, RNAi was known by other names, including co-suppression, post-transcriptional gene silencing (PTGS), and quelling. The detailed study of each of these seemingly different processes elucidated that the identity of these phenomena were all actually RNAi. Andrew Fire and Craig C. Mello shared the 2006 Nobel Prize in Physiology or Medicine for their work on RNAi in the nematode worm Caenorhabditis elegans, which they published in 1998. Since the discovery of RNAi and its regulatory potentials, it has become evident that RNAi has immense potential in suppression of desired genes. RNAi is now known as precise, efficient, stable and better than antisense therapy for gene suppression. Antisense RNA produced intracellularly by an expression vector may be developed and find utility as novel therapeutic agents.
Edelfosine is a synthetic alkyl-lysophospholipid (ALP). It has antineoplastic (anti-cancer) effects.

Gene therapy utilizes the delivery of DNA into cells, which can be accomplished by several methods, summarized below. The two major classes of methods are those that use recombinant viruses and those that use naked DNA or DNA complexes.
Antineoplastic resistance, often used interchangeably with chemotherapy resistance, is the resistance of neoplastic (cancerous) cells, or the ability of cancer cells to survive and grow despite anti-cancer therapies. In some cases, cancers can evolve resistance to multiple drugs, called multiple drug resistance.

Ram I. Mahato is a professor and chairman of the Department of Pharmaceutical Sciences, University of Nebraska Medical Center, Omaha, United States. He was Professor of Pharmaceutical Sciences and Biomedical Engineering at the University of Tennessee Health Science Center, Memphis. He was Research Assistant Professor at the University of Utah, Senior Scientist at GeneMedicine Inc and a postdoctoral fellow at the University of Southern California, Washington University in St. Louis, and Kyoto University. He received a PhD in drug delivery from the University of Strathclyde and BS from China Pharmaceutical University. He is a CRS Fellow, AAPS Fellow, Permanent Member of BTSS/NIH Study section (2009–2013), and ASGCT Scientific Advisor.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.

Cellular adoptive immunotherapy is a type of immunotherapy. Immune cells such as T-cells are usually isolated from patients for expansion or engineering purposes and reinfused back into patients to fight diseases using their own immune system. A major application of cellular adoptive therapy is cancer treatment, as the immune system plays a vital role in the development and growth of cancer. The primary types of cellular adoptive immunotherapies are T cell therapies. Other therapies include CAR-T therapy, CAR-NK therapy, macrophage-based immunotherapy and dendritic cell therapy.
Cytokines are polypeptides or glycoproteins that help immune cells communicate to each other to induce proliferation, activation, differentiation, and inflammatory or anti-inflammatory signals in various cell types. Studies utilizing cytokines for antitumor therapies has increased significantly since 2000, and different cytokines provide unique antitumor activities. Cytokines hinder tumor cell development mostly through antiproliferative or proapoptotic pathways but can also interrupt development indirectly by eliciting immune cells to have cytotoxic effects against tumor cells. Even though there are FDA-approved cytokine therapies, there are two main challenges associated with cytokine delivery. The first is that cytokines have a short half-life, so frequent administration of high doses is required for therapeutic effect. The second is that systemic toxicity could occur if the cytokines delivered cause an intense immune response, known as a cytokine storm.
Reduction-sensitive nanoparticles (RSNP) consist of nanocarriers that are chemically responsive to reduction. Drug delivery systems using RSNP can be loaded with different drugs that are designed to be released within a concentrated reducing environment, such as the tumor-targeted microenvironment. Reduction-Sensitive Nanoparticles provide an efficient method of targeted drug delivery for the improved controlled release of medication within localized areas of the body.












