Related Research Articles

Adenylyl cyclase is an enzyme with key regulatory roles in essentially all cells. It is the most polyphyletic known enzyme: six distinct classes have been described, all catalyzing the same reaction but representing unrelated gene families with no known sequence or structural homology. The best known class of adenylyl cyclases is class III or AC-III. AC-III occurs widely in eukaryotes and has important roles in many human tissues.

Cyclic adenosine monophosphate is a second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway. It should not be confused with 5'-AMP-activated protein kinase.

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.

Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It works to raise the concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism.

The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation. Thus it was thought of as a resting phase. G0 is now known to take different forms and occur for multiple reasons. For example, most adult neuronal cells, among the most metabolically active cells in the body, are fully differentiated and reside in a terminal G0 phase. Neurons reside in this state, not because of stochastic or limited nutrient supply, but as a part of their internal genetic programming.
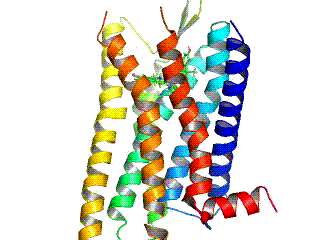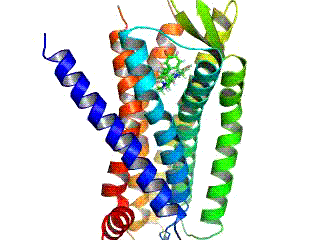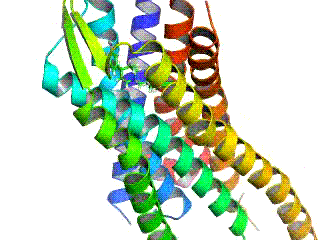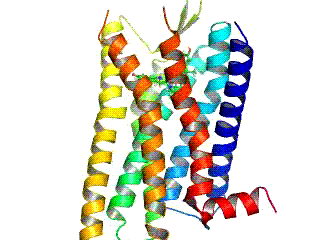
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.

Alfred Goodman Gilman was an American pharmacologist and biochemist. He and Martin Rodbell shared the 1994 Nobel Prize in Physiology or Medicine "for their discovery of G-proteins and the role of these proteins in signal transduction in cells."
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Bordetella bronchiseptica and Bordetella parapertussis, also able to cause pertussis-like symptoms, also produce adenylate cyclase toxin. It is a toxin secreted by the bacteria to influence the host immune system.

Heterotrimeric G protein, also sometimes referred to as the "large" G proteins are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of alpha (α), beta (β) and gamma (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein.
Neuromedin B (NMB) is a bombesin-related peptide in mammals. It was originally purified from pig spinal cord, and later shown to be present in human central nervous system and gastrointestinal tract.

Regulators of G protein signaling (RGS) are protein structural domains or the proteins that contain these domains, that function to activate the GTPase activity of heterotrimeric G-protein α-subunits.

Adenylyl cyclase type 2 is an enzyme typically expressed in the brain of humans, that is encoded by the ADCY2 gene. It belongs to the adenylyl cyclase class-3 or guanylyl cyclase family because it contains two guanylate cyclase domains. ADCY2 is one of ten different mammalian isoforms of adenylyl cyclases. ADCY2 can be found on chromosome 5 and the "MIR2113-POU3F2" region of chromosome 6, with a length of 1091 amino-acids. An essential cofactor for ADCY2 is magnesium; two ions bind per subunit.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
G1/S-specific cyclin Cln3 is a protein that is encoded by the CLN3 gene. The Cln3 protein is a budding yeast G1 cyclin that controls the timing of Start, the point of commitment to a mitotic cell cycle. It is an upstream regulator of the other G1 cyclins, and it is thought to be the key regulator linking cell growth to cell cycle progression. It is a 65 kD, unstable protein; like other cyclins, it functions by binding and activating cyclin-dependent kinase (CDK).

In molecular biology, the cyclase-associated protein family (CAP) is a family of highly conserved actin-binding proteins present in a wide range of organisms including yeast, flies, plants, and mammals. CAPs are multifunctional proteins that contain several structural domains. CAP is involved in species-specific signalling pathways. In Drosophila, CAP functions in Hedgehog-mediated eye development and in establishing oocyte polarity. In Dictyostelium discoideum, CAP is involved in microfilament reorganisation near the plasma membrane in a PIP2-regulated manner and is required to perpetuate the cAMP relay signal to organise fruitbody formation. In plants, CAP is involved in plant signalling pathways required for co-ordinated organ expansion. In yeast, CAP is involved in adenylate cyclase activation, as well as in vesicle trafficking and endocytosis. In both yeast and mammals, CAPs appear to be involved in recycling G-actin monomers from ADF/cofilins for subsequent rounds of filament assembly. In mammals, there are two different CAPs that share 64% amino acid identity.
Whi2 or Whiskey 2 is a 55 kDa globular, cytoplasmatic scaffold protein in Saccharomyces cerevisiae, which plays an essential role in stress response pathways, apparently by passing input signals about nutrient availability on to stress responsive elements and autophagy/mitophagy mechanisms. It is encoded by a 1.46 kbp gene located on chromosome 15.

Eva Julia Neer (1937–2000) was an American physician, biochemist, and cell-biology scientist who gained U.S. national research awards for her discoveries on G-protein subunit structure and function. She described the physiological roles of these subunits as an integrated and versatile molecular system of signal transduction for membrane-receptor regulation of cell function. Her research concepts turned her into a world leader in G-protein studies and impinged widely on the general understanding of cell behavior.
References
- ↑ "RAS2/YNL098C Protein Information". Saccharomyces Genome Database . Retrieved 4 January 2012.
- ↑ Bhattacharya, Sharmila; Chen, Li; Broach, James R.; Powers, Scott (1995). "Ras Membrane Targeting is Essential for Glucose Signaling but not for Viability in Yeast". Proceedings of the National Academy of Sciences. 92 (7): 2984–8. Bibcode:1995PNAS...92.2984B. doi:10.1073/pnas.92.7.2984. JSTOR 2367245. PMC 42343 . PMID 7708760.
- ↑ Leadsham, Jane E.; Miller, Katherine; Ayscough, Kathryn R.; Colombo, Sonia; Martegani, Enzo; Sudbery, Pete; Gourlay, Campbell W. (2009). "Whi2 links nutritional sensing to actin-dependent Ras-cAMP-PKA regulation and apoptosis in yeast". Journal of Cell Science. 122 (5): 706–15. doi:10.1242/jcs.042424. PMC 2720921 . PMID 19208759.
- ↑ Dong, Jian; Bai, Xiaojia (2011). "The membrane localization of Ras2 and the association between Cdc25p and Ras2-GTP are regulated by protein kinase A (PKA) in the yeast Saccharomyces cerevisiae". FEBS Letters. 585 (8): 1127–34. doi: 10.1016/j.febslet.2011.03.057 . PMID 21457714. S2CID 27110.
- ↑ Sadeh, Amit; Movshovich, Natalia; Volokh, Misha; Gheber, Larisa; Aharoni, Amir (2011). "Fine-tuning of the Msn2/4-mediated yeast stress responses as revealed by systematic deletion of Msn2/4 partners". Molecular Biology of the Cell. 22 (17): 3127–38. doi:10.1091/mbc.E10-12-1007. PMC 3164460 . PMID 21757539.