Related Research Articles
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol, or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Glutathione is an organic compound with the chemical formula HOCOCH(NH2)CH2CH2CONHCH(CH2SH)CONHCH2COOH. It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.

In chemistry and biology, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (O2), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH.), and singlet oxygen. ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.
Thioredoxin reductases are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond.
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O2− (superoxide radical), OH (hydroxyl radical) and H2O2 (hydrogen peroxide). Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.

Glutathione reductase (GR) also known as glutathione-disulfide reductase (GSR) is an enzyme that in humans is encoded by the GSR gene. Glutathione reductase catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH), which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell. Glutathione reductase functions as dimeric disulfide oxidoreductase and utilizes an FAD prosthetic group and NADPH to reduce one molar equivalent of GSSG to two molar equivalents of GSH:

Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 in vitro leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome.

Kelch-like ECH-associated protein 1 is a protein that in humans is encoded by the Keap1 gene.

Glutathione peroxidase 2 is an enzyme that in humans is encoded by the GPX2 gene.

Glutaredoxin 2 (GLRX2) is an enzyme that in humans encoded by the GLRX2 gene. GLRX2, also known as GRX2, is a glutaredoxin family protein and a thiol-disulfide oxidoreductase that maintains cellular thiol homeostasis. This gene consists of four exons and three introns, spanned 10 kilobase pairs, and localized to chromosome 1q31.2–31.3.

The reduction-oxidation sensitive green fluorescent protein (roGFP) is a green fluorescent protein engineered to be sensitive to changes in the local redox environment. roGFPs are used as redox-sensitive biosensors.

Bacterial glutathione transferases are part of a superfamily of enzymes that play a crucial role in cellular detoxification. The primary role of GSTs is to catalyze the conjugation of glutathione (GSH) with the electrophilic centers of a wide variety of molecules. The most commonly known substrates of GSTs are xenobiotic synthetic chemicals. There are also classes of GSTs that utilize glutathione as a cofactor rather than a substrate. Often these GSTs are involved in reduction of reactive oxidative species toxic to the bacterium. Conjugation with glutathione receptors renders toxic substances more soluble, and therefore more readily exocytosed from the cell.
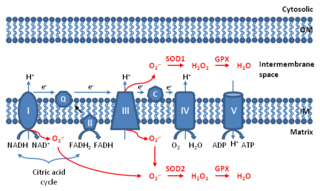
Mitochondrial ROS are reactive oxygen species (ROS) that are produced by mitochondria. Generation of mitochondrial ROS mainly takes place at the electron transport chain located on the inner mitochondrial membrane during the process of oxidative phosphorylation. Leakage of electrons at complex I and complex III from electron transport chains leads to partial reduction of oxygen to form superoxide. Subsequently, superoxide is quickly dismutated to hydrogen peroxide by two dismutases including superoxide dismutase 2 (SOD2) in mitochondrial matrix and superoxide dismutase 1 (SOD1) in mitochondrial intermembrane space. Collectively, both superoxide and hydrogen peroxide generated in this process are considered as mitochondrial ROS.
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.

The mitochondrial theory of ageing has two varieties: free radical and non-free radical. The first is one of the variants of the free radical theory of ageing. It was formulated by J. Miquel and colleagues in 1980 and was developed in the works of Linnane and coworkers (1989). The second was proposed by A. N. Lobachev in 1978.

Sonlicromanol (KH176) is a clinical-stage oral drug compound developed by Khondrion as a potential treatment for inherited mitochondrial diseases, such as Leigh's Disease, MELAS and LHON. Due to dysfunctional mitochondria, an increased level of cellular reactive oxygen species (ROS) is observed in these patients, causing a wide range of symptoms. The active metabolite of Sonlicromanol has several mechanisms of action, acting both as antioxidant and as reactive oxygen species (ROS)-redox modulator. Through selective suppression of microsomal prostaglandin E synthase-1 (mPGES-1), Sonlicromanol even has potency as anti-cancer drug for mPGES-1 overexpressing cancer like prostate cancer. Currently, Sonlicromanol is in phase II clinical trial in the KHENERGYZE, KHENEREXT and KHENERGYC studies as potent candidate in treatment for mitochondrial diseases.

Kenneth D. Tew is a Scottish-American pharmacologist, academic and author. He is a professor in the Department of Cell & Molecular Pharmacology and the John C. West Endowed Chair in Cancer Research at the Medical University of South Carolina.
Danyelle M. Townsend is a biomedical scientist, and academic. She is a Professor and acting Department Chair of Drug Discovery and Biomedical Sciences at the Medical University of South Carolina (MUSC).
References
- 1 2 3 Pérez-Torres, Israel; Guarner-Lans, Verónica; Rubio-Ruiz, María Esther (2017-10-05). "Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents". International Journal of Molecular Sciences. 18 (10): 2098. doi: 10.3390/ijms18102098 . ISSN 1422-0067. PMC 5666780 . PMID 28981461.
- ↑ Xiao, Wusheng; Loscalzo, Joseph (2020-06-20). "Metabolic Responses to Reductive Stress". Antioxidants & Redox Signaling. 32 (18): 1330–1347. doi:10.1089/ars.2019.7803. ISSN 1523-0864. PMC 7247050 . PMID 31218894.
- ↑ Korge, Paavo; Calmettes, Guillaume; Weiss, James N. (2015). "Increased Reactive Oxygen Species Production During Reductive Stress: The Roles of Mitochondrial Glutathione and Thioredoxin Reductases". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1847 (6–7): 514–525. doi:10.1016/j.bbabio.2015.02.012. ISSN 0006-3002. PMC 4426053 . PMID 25701705.
- 1 2 3 Chun, Kyung-Soo; Kim, Do-Hee; Surh, Young-Joon (2021-03-30). "Role of Reductive versus Oxidative Stress in Tumor Progression and Anticancer Drug Resistance". Cells . 10 (4): 758. doi: 10.3390/cells10040758 . ISSN 2073-4409. PMC 8065762 . PMID 33808242.
- ↑ Zhang, Huali; Limphong, Pattraranee; Pieper, Joel; Liu, Qiang; Rodesch, Christopher K.; Christians, Elisabeth; Benjamin, Ivor J. (2012). "Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity". The FASEB Journal . 26 (4): 1442–1451. doi: 10.1096/fj.11-199869 . ISSN 0892-6638. PMC 3316899 . PMID 22202674.
- ↑ Chun, Kyung-Soo; Kim, Do-Hee; Surh, Young-Joon (2021). "Role of Reductive versus Oxidative Stress in Tumor Progression and Anticancer Drug Resistance". Cells . 10 (4): 758. doi: 10.3390/cells10040758 . ISSN 2073-4409. PMC 8065762 . PMID 33808242.
- ↑ Ma, Qiang (2013). "Role of Nrf2 in Oxidative Stress and Toxicity". Annual Review of Pharmacology and Toxicology. 53: 401–426. doi:10.1146/annurev-pharmtox-011112-140320. ISSN 0362-1642. PMC 4680839 . PMID 23294312.
- 1 2 Qiao, Xinhua; Zhang, Yingmin; Ye, Aojun; Zhang, Yini; Xie, Ting; Lv, Zhenyu; Wu, Xun; Zhang, Weiqi; Wang, Ping; Liu, Guang-Hui; Wang, Chih-chen (2021). "Reductive Stress in the Endoplasmic Reticulum Caused by Ero1α S-Nitrosation Accelerates Senescence". SSRN Electronic Journal . doi:10.2139/ssrn.3869890. ISSN 1556-5068. S2CID 237913085.
- ↑ Handy, Diane E.; Loscalzo, Joseph (2017). "Responses to reductive stress in the cardiovascular system". Free Radical Biology and Medicine . 109: 114–124. doi:10.1016/j.freeradbiomed.2016.12.006. PMC 5462861 . PMID 27940350.
- ↑ Zhang, Xia; Min, Xiaoyan; Li, Chuanfu; Benjamin, Ivor J.; Qian, Bo; Zhang, Xiaojin; Ding, Zhengnian; Gao, Xiang; Yao, Yuzhen; Ma, Yujie; Cheng, Yunling; Liu, Li (2010-06-01). "Involvement of Reductive Stress in the Cardiomyopathy in Transgenic Mice With Cardiac-Specific Overexpression of Heat Shock Protein 27". Hypertension. 55 (6): 1412–1417. doi: 10.1161/HYPERTENSIONAHA.109.147066 . PMID 20439823. S2CID 13429934.