
In genetics, complementary DNA (cDNA) is DNA synthesized from a single-stranded RNA template in a reaction catalyzed by the enzyme reverse transcriptase. cDNA is often used to clone eukaryotic genes in prokaryotes. When scientists want to express a specific protein in a cell that does not normally express that protein, they will transfer the cDNA that codes for the protein to the recipient cell. In molecular biology, cDNA is also generated to analyze transcriptomic profiles in bulk tissue, single cells, or single nuclei in assays such as microarrays and RNA-seq.
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physical structure of biological macromolecules is known as molecular biology.
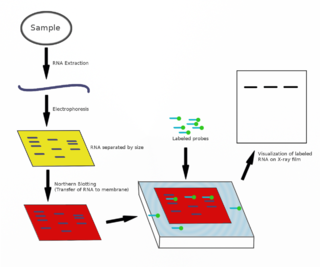
The northern blot, or RNA blot, is a technique used in molecular biology research to study gene expression by detection of RNA in a sample.

A Southern blot is a method used in molecular biology for detection of a specific DNA sequence in DNA samples. Southern blotting combines transfer of electrophoresis-separated DNA fragments to a filter membrane and subsequent fragment detection by probe hybridization.

Reverse transcription polymerase chain reaction (RT-PCR) is a laboratory technique combining reverse transcription of RNA into DNA and amplification of specific DNA targets using polymerase chain reaction (PCR). It is primarily used to measure the amount of a specific RNA. This is achieved by monitoring the amplification reaction using fluorescence, a technique called real-time PCR or quantitative PCR (qPCR). Combined RT-PCR and qPCR are routinely used for analysis of gene expression and quantification of viral RNA in research and clinical settings.

A DNA microarray is a collection of microscopic DNA spots attached to a solid surface. Scientists use DNA microarrays to measure the expression levels of large numbers of genes simultaneously or to genotype multiple regions of a genome. Each DNA spot contains picomoles of a specific DNA sequence, known as probes. These can be a short section of a gene or other DNA element that are used to hybridize a cDNA or cRNA sample under high-stringency conditions. Probe-target hybridization is usually detected and quantified by detection of fluorophore-, silver-, or chemiluminescence-labeled targets to determine relative abundance of nucleic acid sequences in the target. The original nucleic acid arrays were macro arrays approximately 9 cm × 12 cm and the first computerized image based analysis was published in 1981. It was invented by Patrick O. Brown. An example of its application is in SNPs arrays for polymorphisms in cardiovascular diseases, cancer, pathogens and GWAS analysis. It is also used for the identification of structural variations and the measurement of gene expression.
The transcriptome is the set of all RNA transcripts, including coding and non-coding, in an individual or a population of cells. The term can also sometimes be used to refer to all RNAs, or just mRNA, depending on the particular experiment. The term transcriptome is a portmanteau of the words transcript and genome; it is associated with the process of transcript production during the biological process of transcription.
This is a list of topics in molecular biology. See also index of biochemistry articles.
In molecular biology, a hybridization probe(HP) is a fragment of DNA or RNA of usually 15–10000 nucleotide long which can be radioactively or fluorescently labeled. HP can be used to detect the presence of nucleotide sequences in analyzed RNA or DNA that are complementary to the sequence in the probe. The labeled probe is first denatured into single stranded DNA (ssDNA) and then hybridized to the target ssDNA or RNA immobilized on a membrane or in situ.
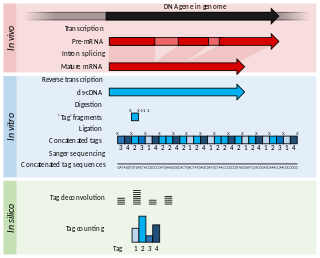
Serial Analysis of Gene Expression (SAGE) is a transcriptomic technique used by molecular biologists to produce a snapshot of the messenger RNA population in a sample of interest in the form of small tags that correspond to fragments of those transcripts. Several variants have been developed since, most notably a more robust version, LongSAGE, RL-SAGE and the most recent SuperSAGE. Many of these have improved the technique with the capture of longer tags, enabling more confident identification of a source gene.
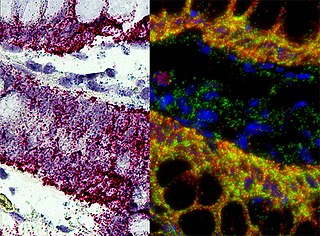
In situ hybridization (ISH) is a type of hybridization that uses a labeled complementary DNA, RNA or modified nucleic acids strand to localize a specific DNA or RNA sequence in a portion or section of tissue or if the tissue is small enough, in the entire tissue, in cells, and in circulating tumor cells (CTCs). This is distinct from immunohistochemistry, which usually localizes proteins in tissue sections.
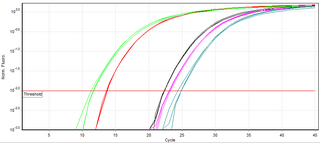
A real-time polymerase chain reaction is a laboratory technique of molecular biology based on the polymerase chain reaction (PCR). It monitors the amplification of a targeted DNA molecule during the PCR, not at its end, as in conventional PCR. Real-time PCR can be used quantitatively and semi-quantitatively.
An RNA spike-in is an RNA transcript of known sequence and quantity used to calibrate measurements in RNA hybridization assays, such as DNA microarray experiments, RT-qPCR, and RNA-Seq.
Subtractive hybridization is a technology that allows for PCR-based amplification of only cDNA fragments that differ between a control (driver) and experimental transcriptome. cDNA is produced from mRNA. Differences in relative abundance of transcripts are highlighted, as are genetic differences between species. The technique relies on the removal of dsDNA formed by hybridization between a control and test sample, thus eliminating cDNAs or gDNAs of similar abundance, and retaining differentially expressed, or variable in sequence, transcripts or genomic sequences.
An allele-specific oligonucleotide (ASO) is a short piece of synthetic DNA complementary to the sequence of a variable target DNA. It acts as a probe for the presence of the target in a Southern blot assay or, more commonly, in the simpler Dot blot assay. It is a common tool used in genetic testing, forensics, and Molecular Biology research.
Differential display is a laboratory technique that allows a researcher to compare and identify changes in gene expression at the mRNA level between two or more eukaryotic cell samples. It was the most commonly used method to compare expression profiles of two eukaryotic cell samples in the 1990s. By 2000, differential display was superseded by DNA microarray approaches.

MAGIChips, also known as "microarrays of gel-immobilized compounds on a chip" or "three-dimensional DNA microarrays", are devices for molecular hybridization produced by immobilizing oligonucleotides, DNA, enzymes, antibodies, and other compounds on a photopolymerized micromatrix of polyacrylamide gel pads of 100x100x20µm or smaller size. This technology is used for analysis of nucleic acid hybridization, specific binding of DNA, and low-molecular weight compounds with proteins, and protein-protein interactions.
Massive parallel signature sequencing (MPSS) is a procedure that is used to identify and quantify mRNA transcripts, resulting in data similar to serial analysis of gene expression (SAGE), although it employs a series of biochemical and sequencing steps that are substantially different.
Transcriptomics technologies are the techniques used to study an organism's transcriptome, the sum of all of its RNA transcripts. The information content of an organism is recorded in the DNA of its genome and expressed through transcription. Here, mRNA serves as a transient intermediary molecule in the information network, whilst non-coding RNAs perform additional diverse functions. A transcriptome captures a snapshot in time of the total transcripts present in a cell. Transcriptomics technologies provide a broad account of which cellular processes are active and which are dormant. A major challenge in molecular biology is to understand how a single genome gives rise to a variety of cells. Another is how gene expression is regulated.

Spatial transcriptomics is a method for assigning cell types to their locations in the histological sections. This method can also be used to determine subcellular localization of mRNA molecules. The term is a variation of Spatial Genomics, first described by Doyle, et al., in 2000 and then expanded upon by Ståhl et al. in a technique developed in 2016, which has since undergone a variety of improvements and modifications.











