
Apoptosis is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses 50 to 70 billion cells each day due to apoptosis. For the average human child between 8 and 14 years old, each day the approximate loss is 20 to 30 billion cells.

Caspases are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions.
Programmed cell death is the death of a cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers advantage during an organism's lifecycle. For example, the differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and animal tissue development.

Howard Robert Horvitz ForMemRS NAS AAA&S APS NAM is an American biologist whose research on the nematode worm Caenorhabditis elegans was awarded the 2002 Nobel Prize in Physiology or Medicine, together with Sydney Brenner and John E. Sulston, whose "seminal discoveries concerning the genetic regulation of organ development and programmed cell death" were "important for medical research and have shed new light on the pathogenesis of many diseases".

Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, as in programmed cell death, or may result from factors such as diseases, localized injury, or the death of the organism of which the cells are part. Apoptosis or Type I cell-death, and autophagy or Type II cell-death are both forms of programmed cell death, while necrosis is a non-physiological process that occurs as a result of infection or injury.

The death-effector domain (DED) is a protein interaction domain found only in eukaryotes that regulates a variety of cellular signalling pathways. The DED domain is found in inactive procaspases and proteins that regulate caspase activation in the apoptosis cascade such as FAS-associating death domain-containing protein (FADD). FADD recruits procaspase 8 and procaspase 10 into a death induced signaling complex (DISC). This recruitment is mediated by a homotypic interaction between the procaspase DED and a second DED that is death effector domain in an adaptor protein that is directly associated with activated TNF receptors. Complex formation allows proteolytic activation of procaspase into the active caspase form which results in the initiation of apoptosis. Structurally the DED domain are a subclass of protein motif known as the death fold and contains 6 alpha helices, that closely resemble the structure of the Death domain (DD).
Guy Salvesen is a South African-born biochemist, best known for his work in the field of apoptosis. His research focuses on proteases and their inhibitors in humans, with particular emphasis on the caspases of the apoptotic cell death pathway.
Phenoptosis is a conception of the self-programmed death of an organism proposed by Vladimir Skulachev in 1999.

Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5, is a protein that, in humans, is encoded by the BIRC5 gene.
Apoptosis is the process of programmed cell death. From its early conceptual beginnings in the 1950s, it has exploded as an area of research within the life sciences community. As well as its implication in many diseases, it is an integral part of biological development.

Death-associated protein 6 also known as Daxx is a protein that in humans is encoded by the DAXX gene.

Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the CASP3 gene. CASP3 orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts.
In cellular biology, dependence receptors are proteins that mediate programmed cell death by monitoring the absence of certain trophic factors that otherwise serve as ligands (interactors) for the dependence receptors. A trophic ligand is a molecule whose protein binding stimulates cell growth, differentiation, and/or survival. Cells depend for their survival on stimulation that is mediated by various receptors and sensors, and integrated via signaling within the cell and between cells. The withdrawal of such trophic support leads to a form of cellular suicide.
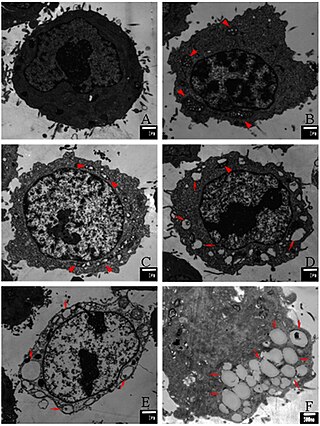
Paraptosis is a type of programmed cell death, morphologically distinct from apoptosis and necrosis. The defining features of paraptosis are cytoplasmic vacuolation, independent of caspase activation and inhibition, and lack of apoptotic morphology. Paraptosis lacks several of the hallmark characteristics of apoptosis, such as membrane blebbing, chromatin condensation, and nuclear fragmentation. Like apoptosis and other types of programmed cell death, the cell is involved in causing its own death, and gene expression is required. This is in contrast to necrosis, which is non-programmed cell death that results from injury to the cell.
Emad Saleem Alnemri is a professor in Biochemistry & Molecular Biology at the Sidney Kimmel Cancer Center, Thomas Jefferson University, researching apoptosis and the inflammasome.
Ted M. Dawson is an American neurologist and neuroscientist. He is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases and Director of the Institute for Cell Engineering at Johns Hopkins University School of Medicine. He has joint appointments in the Department of Neurology, Neuroscience and Department of Pharmacology and Molecular Sciences.
Patrick Mehlen, is a French biologist and research director at the Centre national de recherche scientifique (CNRS) at the Centre Léon-Bérard, a cancer research centre in Lyon.

Death regulator Nedd2-like caspase was firstly identified and characterised in Drosophila in 1999 as a cysteine protease containing an amino-terminal caspase recruitment domain. At first, it was thought of as an effector caspase involved in apoptosis, but subsequent findings have proved that it is, in fact, an initiator caspase with a crucial role in said type of programmed cell death.
Alfred Lewis Goldberg was an American cell biologist-biochemist and professor at Harvard University. His major discoveries have concerned the mechanisms and physiological importance of protein degradation in cells. Of wide impact have been his lab's demonstration that all cells contain a pathway for selectively eliminating misfolded proteins, his discoveries about the role of proteasomes in this process and of the enzyme systems catalyzing protein breakdown in bacteria, his elucidating the mechanisms for muscle atrophy and the role of proteasomes in antigen presentation to the immune system, and his introduction of proteasome inhibitors now widely used as research tools and in the treatment of blood cancers.
Seamus J. Martin is an Irish molecular biologist and immunologist working at The Smurfit Institute of Genetics in Trinity College Dublin. Since 1999, he has held the Smurfit Chair of Medical Genetics at Trinity College Dublin, and his research focuses on the links between cell death, cell stress, and inflammation. Martin is known for his contributions to understanding the molecular control of the mode of regulated cell death known as apoptosis. Martin received the 'GlaxoSmithKline Award' of the Biochemical Society in 2006, the British Science Association's 'Charles Darwin Award' in 2005, and The 'RDS-Irish Times Boyle Medal' in 2014, for his work on deciphering the role of caspases in apoptosis. In 2006, he was elected to the Royal Irish Academy, in 2009 he awarded EMBO Membership, and in 2023 he was elected to the Academia Europaea. His research work is widely cited and he received a European Research Council Advanced Research award in 2021.










