Related Research Articles

A coccolithophore is a unicellular, eukaryotic phytoplankton (alga). Coccolithophores belong either to the kingdom Protista, according to Robert Whittaker's Five kingdom classification, or clade Hacrobia, according to the newer biological classification system. Within the Hacrobia, the coccolithophores are in the phylum or division Haptophyta, class Prymnesiophyceae. Coccolithophores are distinguished by special calcium carbonate plates of uncertain function called coccoliths, which are also important microfossils. However, there are Prymnesiophyceae species lacking coccoliths, so not every member of Prymnesiophyceae is a coccolithophore. Coccolithophores are almost exclusively marine, are photosynthetic, and exist in large numbers throughout the sunlight zone of the ocean.

In oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.
Marine pollution occurs when substances used or spread by humans, such as industrial, agricultural and residential waste, particles, noise, excess carbon dioxide or invasive organisms enter the ocean and cause harmful effects there. The majority of this waste (80%) comes from land-based activity, although marine transportation significantly contributes as well. Since most inputs come from land, either via the rivers, sewage or the atmosphere, it means that continental shelves are more vulnerable to pollution. Air pollution is also a contributing factor by carrying off iron, carbonic acid, nitrogen, silicon, sulfur, pesticides or dust particles into the ocean. The pollution often comes from nonpoint sources such as agricultural runoff, wind-blown debris, and dust. These nonpoint sources are largely due to runoff that enters the ocean through rivers, but wind-blown debris and dust can also play a role, as these pollutants can settle into waterways and oceans. Pathways of pollution include direct discharge, land runoff, ship pollution, atmospheric pollution and, potentially, deep sea mining.
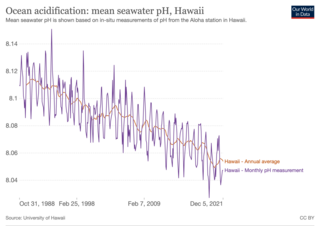
Ocean acidification is the reduction in the pH of the Earth’s ocean. This process takes place over periods lasting decades or more. Its main cause is the absorption of carbon dioxide (CO2) from the atmosphere. This, in turn, increases CO2 concentrations in the ocean. Between 23 and 30% of the CO2 that is in the atmosphere dissolves into oceans, rivers and lakes. Acidification is one of several effects of rising CO2 on the ocean. Other chemical changes to the ocean can also cause acidification. As the ocean absorbs CO2, seawater chemistry changes, which changes the living conditions of marine species. Many different species are affected, especially organisms that rely on calcium carbonate shells and skeletons, like mollusks, oysters and corals. Organisms like these struggle to build those parts of their anatomy when ocean waters have increased acidity.
The Global Ocean Data Analysis Project (GLODAP) is a synthesis project bringing together oceanographic data, featuring two major releases as of 2018. The central goal of GLODAP is to generate a global climatology of the World Ocean's carbon cycle for use in studies of both its natural and anthropogenically forced states. GLODAP is funded by the National Oceanic and Atmospheric Administration, the U.S. Department of Energy, and the National Science Foundation.

Rhodoliths are colorful, unattached calcareous nodules, composed of crustose, benthic marine red algae that resemble coral. Rhodolith beds create biogenic habitat for diverse benthic communities. The rhodolithic growth habit has been attained by a number of unrelated coralline red algae, organisms that deposit calcium carbonate within their cell walls to form hard structures or nodules that resemble beds of coral.

A wild fishery is a natural body of water with a sizeable free-ranging fish or other aquatic animal population that can be harvested for its commercial value. Wild fisheries can be marine (saltwater) or lacustrine/riverine (freshwater), and rely heavily on the carrying capacity of the local aquatic ecosystem.

Human activities have significant impact on coral reefs. Coral reefs are dying around the world. Damaging activities include coral mining, pollution, overfishing, blast fishing, the digging of canals and access into islands and bays. Other threats include disease, destructive fishing practices and warming oceans. The ocean's role as a carbon dioxide sink, atmospheric changes, ultraviolet light, ocean acidification, viruses, impacts of dust storms carrying agents to far-flung reefs, pollutants, algal blooms are some of the factors that affect coral reefs. Evidently, coral reefs are threatened well beyond coastal areas. Climate change, such as global warming causes coral bleaching which can be fatal to the corals.

There are many significant effects of climate change on oceans including: an increase in sea surface temperature as well as ocean temperatures at greater depths, more frequent marine heatwaves, a reduction in pH value, a rise in sea level from ocean warming and ice sheet melting, sea ice decline in the Arctic, increased upper ocean stratification, reductions in oxygen levels, increased contrasts in salinity, changes to ocean currents including a weakening of the Atlantic meridional overturning circulation, and stronger tropical cyclones and monsoons. All these changes have knock-on effects which disturb marine ecosystems. The root cause of these observed changes is the Earth warming due to anthropogenic emissions of greenhouse gases, such as for example carbon dioxide and methane. This leads inevitably to ocean warming, because the ocean is taking up most of the additional heat in the climate system. Some of the additional carbon dioxide in the atmosphere is taken up by the ocean, which leads to ocean acidification of the ocean water. It is estimated that the ocean takes up roughly a quarter of total anthropogenic CO2 emissions.
Estuarine acidification happens when the pH balance of water in coastal marine ecosystems, specifically those of estuaries, decreases. Water, generally considered neutral on the pH scale, normally perfectly balanced between alkalinity and acidity. While ocean acidification occurs due to the ongoing decrease in the pH of the Earth's oceans, caused by the absorption of carbon dioxide (CO2) from the atmosphere, pH change in estuaries is more complicated than in the open ocean due to direct impacts from land run-off, human impact, and coastal current dynamics. In the ocean, wave and wind movement allows carbon dioxide (CO2) to mixes with water (H2O) forming carbonic acid (H2CO3). Through wave motion this chemical bond is mixed up, allowing for the further break of the bond, eventually becoming carbonate (CO3) which is basic and helps form shells for ocean creatures, and two hydron molecules. This creates the potential for acidic threat since hydron ions readily bond with any Lewis Structure to form an acidic bond. This is referred to as an oxidation-reduction reaction.

Ocean acidification threatens the Great Barrier Reef by reducing the viability and strength of coral reefs. The Great Barrier Reef, considered one of the seven natural wonders of the world and a biodiversity hotspot, is located in Australia. Similar to other coral reefs, it is experiencing degradation due to ocean acidification. Ocean acidification results from a rise in atmospheric carbon dioxide, which is taken up by the ocean. This process can increase sea surface temperature, decrease aragonite, and lower the pH of the ocean. The more humanity consumes fossil fuels, the more the ocean absorbs released CO₂, furthering ocean acidification.

Justin Baker Ries is an American marine scientist, best known for his contributions to ocean acidification, carbon sequestration, and biomineralization research.

Tessa Michelle Hill is an American marine geochemist and oceanographer. She is a professor at the University of California, Davis, and a resident professor at its Bodega Marine Laboratory. She is a Fellow of the California Academy of Sciences, and in 2016 was named a Leshner Public Engagement Fellow of the American Association for the Advancement of Science. In that year she also received the US Presidential Early Career Award for Scientists and Engineers (PECASE).

The Arctic ocean covers an area of 14,056,000 squared kilometers, and supports a diverse and important socioeconomic food web of organisms, despite its average water temperature being 32 degrees Fahrenheit. Over the last three decades, the Arctic Ocean has experienced drastic changes due to climate change. One of the changes is in the acidity levels of the ocean, which have been consistently increasing at twice the rate of the Pacific and Atlantic oceans. Arctic Ocean acidification is a result of feedback from climate system mechanisms, and is having negative impacts on Arctic Ocean ecosystems and the organisms that live within them.

Human activities affect marine life and marine habitats through overfishing, habitat loss, the introduction of invasive species, ocean pollution, ocean acidification and ocean warming. These impact marine ecosystems and food webs and may result in consequences as yet unrecognised for the biodiversity and continuation of marine life forms.

Jean-Pierre Gattuso is a French ocean scientist conducting research globally, from the pole to the tropics and from nearshore to the open ocean. His research addresses the biology of reef-building corals, the biogeochemistry of coastal ecosystems, and the response of marine plants, animals and ecosystems to global environmental change. He is also interested in transdisciplinary research, collaborating with social scientists to address ocean-based solutions to minimize climate change and its impacts. He is currently a CNRS Research Professor at Sorbonne University.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.
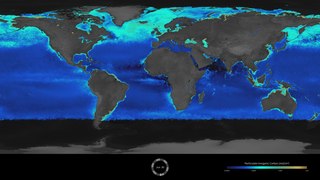
The Great Calcite Belt (GCB) of the Southern Ocean is a region of elevated summertime upper ocean calcite concentration derived from coccolithophores, despite the region being known for its diatom predominance. The overlap of two major phytoplankton groups, coccolithophores and diatoms, in the dynamic frontal systems characteristic of this region provides an ideal setting to study environmental influences on the distribution of different species within these taxonomic groups.
Scott Doney is a marine scientist at the University of Virginia known for his work on biogeochemical modeling. Doney is the Joe D. and Helen J. Kington Professor in Environmental Change, a fellow of the American Geophysical Union, the American Association for the Advancement of Science., and the Association for the Sciences of Limnology and Oceanography. He is currently serving as the Assistant Director for Ocean Climate Science and Policy in the White House Office of Science and Technology Policy.
Parker MacCready is an American oceanographer. He is a professor at the School of Oceanography at the University of Washington.
References
- ↑ "AAAS Names 8 UW Researchers as Fellows in 2017". washington.edu. November 22, 2017. Retrieved December 22, 2017.
- 1 2 3 "Richard A. Feely". noaa.gov. Retrieved December 22, 2017.
- ↑ "AAAS Names 8 UW Researchers as Fellows in 2017". washington.edu. November 22, 2017. Retrieved December 22, 2017.
- ↑ James C Orr, Victoria J Fabry, Olivier Aumont, Laurent Bopp, Scott C Doney, Richard A Feely, Anand Gnanadesikan, Nicolas Gruber, Akio Ishida, Fortunat Joos, Robert M Key, Keith Lindsay, Ernst Maier-Reimer, Richard Matear, Patrick Monfray, Anne Mouchet, Raymond G Najjar, Gian-Kasper Plattner, Keith B Rodgers, Christopher L Sabine, Jorge L Sarmiento, Reiner Schlitzer, Richard D Slater, Ian J Totterdell, Marie-France Weirig, Yasuhiro Yamanaka, Andrew Yool. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. 437: 7059. 681-686. Nature. 2005
- ↑ "Richard Feely" . Retrieved December 22, 2017.