Related Research Articles

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.

Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used. In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses. By 2007, it was only used in the treatment of prostate cancer and breast cancer. In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer. While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.
Bioidentical hormone replacement therapy (BHRT), also known as bioidentical hormone therapy (BHT) or natural hormone therapy, is the use of hormones that are identical on a molecular level with endogenous hormones in hormone replacement therapy. It may also be combined with blood and saliva testing of hormone levels, and the use of pharmacy compounding to obtain hormones in an effort to reach a targeted level of hormones in the body. A number of claims by some proponents of BHT have not been confirmed through scientific testing. Specific hormones used in BHT include estrone, estradiol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and estriol.

Estradiol acetate (EA), sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women. It is taken by mouth once daily or given as a vaginal ring once every three months.
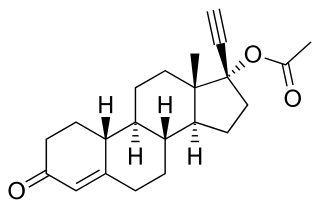
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. Effects of menopause can include symptoms such as hot flashes, accelerated skin aging, vaginal dryness, decreased muscle mass, and complications such as osteoporosis, sexual dysfunction, and vaginal atrophy. They are mostly caused by low levels of female sex hormones that occur during menopause.

Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders. It is also used in the treatment of prostate cancer in men. Estradiol benzoate is used in veterinary medicine as well. When used clinically, the medication is given by injection into muscle usually two to three times per week.
Vaginal estrogen is a form of estrogen that is delivered by intravaginal administration. Vaginally administered estrogens are thereby exerting their effects mainly in the nearby tissue, with more limited systemic effects compared to orally administered estrogens. It will not protect against osteoporosis. With perhaps the exception of the Femring, it also will not alleviate the hot flashes and hormonal imbalance caused by menopause.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Mytatrienediol, also known as 16α-methyl-16β-epiestriol 3-methyl ether or 16β-hydroxy-16α-methylestradiol 3-methyl ether, is a synthetic steroidal estrogen medication and an estrogen ether which was derived from estriol and was developed for clinical use in the late 1950s but was never marketed. It was investigated as a weak and mildly estrogenic medication for men to treat atherosclerosis, improve serum lipid profiles, and reduce the risk of myocardial infarction. However, while preclinical research supported the profile of mytatriendiol as a weak estrogen, the medication was found in clinical trials to produce estrogenic side effects including feminization, breast pain, and gynecomastia in men similarly and comparably to other estrogens such as ethinylestradiol and conjugated estrogens, and its side effects ultimately precluded its use. The medication was also studied to treat bone pain in patients with multiple myeloma, metastatic bone disease, and osteoporosis, with effectiveness seen.

Clomestrone, also known as 16α-chloroestrone 3-methyl ether, is a synthetic, steroidal, weak estrogen derived from estrone and used as an anticholesterolemic agent in the treatment of atherosclerosis. It is said to have beneficial effects on serum lipid profiles while producing minimal feminization, though some estrogenic side effects, including breast tenderness, loss of libido, and fatigue or avolition, were observed in most patients in clinical studies. The drug is a close analogue of mytatrienediol, and the two estrogens have similar drug profiles. Clomestrone was described in the literature in 1958 and introduced for medical use shortly thereafter.

Estriol glucuronide (E3G), or oestriol glucuronide, also known as estriol monoglucuronide, as well as estriol 16α-β-D-glucosiduronic acid, is a natural, steroidal estrogen and the glucuronic acid conjugate of estriol. It occurs in high concentrations in the urine of pregnant women as a reversibly formed metabolite of estriol. Estriol glucuronide is a prodrug of estriol, and was the major component of Progynon and Emmenin, estrogenic products manufactured from the urine of pregnant women that were introduced in the 1920s and 1930s and were the first orally active estrogens. Emmenin was succeeded by Premarin, which is sourced from the urine of pregnant mares and was introduced in 1941. Premarin replaced Emmenin due to the fact that it was easier and less expensive to produce.

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in feminizing hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.

Conjugated estrogens/methyltestosterone (CEEs/MT), sold under the brand name Premarin with Methyltestosterone, is a combination of conjugated estrogens (CEEs), an estrogen, and methyltestosterone (MT), an androgen and anabolic steroid (AAR), which is used in menopausal hormone therapy for women. It contains 0.625 to 1.25 mg CEEs and 5 to 10 mg MT. The medication was marketed by Wyeth-Ayerst. CEEs/MT was previously marketed in the United States and Canada. It remains available only in Paraguay, under the brand names Delitan and Delitan Forte.

Feminine Forever is a 1966 book written by American gynecologist Robert A. Wilson. The book characterized menopause and associated symptoms as a serious disease state and strongly advocated the use of estrogen-based menopausal hormone therapy to alleviate it, maintain femininity and well-being, and improve quality of life and health. Wilson's claims were criticized as not being based on adequate research and evidence. Nonetheless, Wilson's book was marketed directly to women and was a best-seller, with it having been implicated in causing a rapid and large rise in prescriptions of menopausal hormone therapy. Subsequently, trials such as the Women's Health Initiative (WHI) contradicted Wilson's claims and showed that menopausal hormone therapy could have significant medical risks, such as venous thromboembolism and breast cancer, and that its benefits were not as great as once believed.
References
- 1 2 3 4 5 Houck, Judith A. (31 December 2006). "7. Feminine Forever: Robert A. Wilson and the Hormonal Revolution, 1963–1980". Hot and Bothered: Women, Medicine, and Menopause in Modern America. Harvard University Press. pp. 152–187. doi:10.4159/9780674038813-008.
- 1 2 3 4 5 6 Elizabeth Siegel Watkins (16 April 2007). The Estrogen Elixir: A History of Hormone Replacement Therapy in America. JHU Press. ISBN 978-0-8018-8602-7. OCLC 237124873.
- ↑ "Hormone Replacement Study A Shock to the Medical System". The New York Times. 10 July 2002. Retrieved 4 April 2023.
- ↑ Daniel Lee Kleinman; Abby J. Kinchy; Jo Handelsman, eds. (6 May 2005). Controversies in Science and Technology: From Maize to Menopause. Univ of Wisconsin Press. pp. 210–. ISBN 978-0-299-20393-1. OCLC 1018085039.
- ↑ Dominus, Susan (2023-02-01). "Women Have Been Misled About Menopause". The New York Times. ISSN 0362-4331 . Retrieved 2023-02-07.
Every woman has the right — indeed the duty — to counteract the chemical castration that befalls her during her middle years," the gynecologist Robert Wilson wrote in 1966. The U.S. Food and Drug Administration approved the first hormone-therapy drug in 1942, but Wilson's blockbuster book, "Feminine Forever," can be considered a kind of historical landmark...Within a decade of the book's publication, Premarin — a mix of estrogens derived from the urine of pregnant horses — was the fifth-most-prescribed drug in the United States. (Decades later, it was revealed that Wilson received funding from the pharmaceutical company that sold Premarin.)