
An insulin pump is a medical device used for the administration of insulin in the treatment of diabetes mellitus, also known as continuous subcutaneous insulin therapy. The device configuration may vary depending on design. A traditional pump includes:
Intensive insulin therapy or flexible insulin therapy is a therapeutic regimen for diabetes mellitus treatment. This newer approach contrasts with conventional insulin therapy. Rather than minimize the number of insulin injections per day, the intensive approach favors flexible meal times with variable carbohydrate as well as flexible physical activities. The trade-off is the increase from 2 or 3 injections per day to 4 or more injections per day, which was considered "intensive" relative to the older approach. In North America in 2004, many endocrinologists prefer the term "flexible insulin therapy" (FIT) to "intensive therapy" and use it to refer to any method of replacing insulin that attempts to mimic the pattern of small continuous basal insulin secretion of a working pancreas combined with larger insulin secretions at mealtimes. The semantic distinction reflects changing treatment.

Diabetic ketoacidosis (DKA) is a potentially life-threatening complication of diabetes mellitus. Signs and symptoms may include vomiting, abdominal pain, deep gasping breathing, increased urination, weakness, confusion and occasionally loss of consciousness. A person's breath may develop a specific "fruity" smell. The onset of symptoms is usually rapid. People without a previous diagnosis of diabetes may develop DKA as the first obvious symptom.

Blood glucose monitoring is the use of a glucose meter for testing the concentration of glucose in the blood (glycemia). Particularly important in diabetes management, a blood glucose test is typically performed by piercing the skin to draw blood, then applying the blood to a chemically active disposable 'test-strip'. The other main option is continuous glucose monitoring (CGM). Different manufacturers use different technology, but most systems measure an electrical characteristic and use this to determine the glucose level in the blood. Skin-prick methods measure capillary blood glucose, whereas CGM correlates interstitial fluid glucose level to blood glucose level. Measurements may occur after fasting or at random nonfasting intervals, each of which informs diagnosis or monitoring in different ways.

Diabetic hypoglycemia is a low blood glucose level occurring in a person with diabetes mellitus. It is one of the most common types of hypoglycemia seen in emergency departments and hospitals. According to the National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP), and based on a sample examined between 2004 and 2005, an estimated 55,819 cases involved insulin, and severe hypoglycemia is likely the single most common event.

Tolbutamide is a first-generation potassium channel blocker, sulfonylurea oral hypoglycemic medication. This drug may be used in the management of type 2 diabetes if diet alone is not effective. Tolbutamide stimulates the secretion of insulin by the pancreas.

Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that occurs when pancreatic are destroyed by the body's immune system. In healthy persons, beta cells produce insulin. Insulin is a hormone required by the body to store and convert blood sugar into energy. T1D results in high blood sugar levels in the body prior to treatment. Common symptoms include frequent urination, increased thirst, increased hunger, weight loss, and other complications. Additional symptoms may include blurry vision, tiredness, and slow wound healing. While some cases take longer, symptoms usually appear within weeks or a few months.
The main goal of diabetes management is to keep blood glucose (BG) levels as normal as possible. If diabetes is not well controlled, further challenges to health may occur. People with diabetes can measure blood sugar by various methods, such as with a BG meter or a continuous glucose monitor, which monitors over several days. Glucose can also be measured by analysis of a routine blood sample. Usually, people are recommended to control diet, exercise, and maintain a healthy weight, although some people may need medications to control their blood sugar levels. Other goals of diabetes management are to prevent or treat complications that can result from the disease itself and from its treatment.
An insulin analog is any of several types of medical insulin that are altered forms of the hormone insulin, different from any occurring in nature, but still available to the human body for performing the same action as human insulin in terms of controlling blood glucose levels in diabetes. Through genetic engineering of the underlying DNA, the amino acid sequence of insulin can be changed to alter its ADME characteristics. Officially, the U.S. Food and Drug Administration (FDA) refers to these agents as insulin receptor ligands, although they are usually just referred to as insulin analogs or even just insulin.
Pulsatile intravenous insulin therapy, sometimes called metabolic activation therapy or cellular activation therapy, describes in a literal sense the intravenous injection of insulin in pulses versus continuous infusions. Injection of insulin in pulses mimics the physiological secretions of insulin by the pancreas into the portal vein which then drains into the liver. In healthy, non-diabetic individuals, pancreatic secretions of insulin correspond to the intake of food. The pancreas will secrete variable amounts of insulin based upon the amount of food consumed among other factors. Continuous exposure to insulin and glucagon is known to decrease the hormones' metabolic effectiveness on glucose production in humans due to the body developing an increased tolerance to the hormones. Down-regulation at the cellular level may partially explain the decreased action of steady-state levels of insulin, while pulsatile hormone secretion may allow recovery of receptor affinity and numbers for insulin. Intermittent intravenous insulin administration with peaks of insulin concentrations may enhance suppression of gluconeogenesis and reduce hepatic glucose production.

Automated insulin delivery systems are automated systems designed to assist people with insulin-requiring diabetes, by automatically adjusting insulin delivery in response to blood glucose levels. Currently available systems can only deliver a single hormone—insulin. Other systems currently in development aim to improve on current systems by adding one or more additional hormones that can be delivered as needed, providing something closer to the endocrine functionality of the pancreas.
The untethered regimen is a technique combining the use of an insulin pump with a slow-acting insulin analog such as Lantus or Levemir. This allows an insulin dependent person to disconnect the pump when desired while maintaining the flexible benefits that the insulin pump can provide.

Ken Strauss is a physician/author currently living in Spain. He is an Internist and Endocrinologist. For three decades he was Global Medical Director for BD, a leading medical and diagnostic enterprise. He is also Director for Safety in Medicine at the European Medical Association in Brussels.
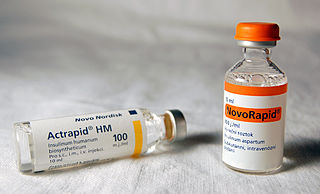
As a medication, insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Such conditions include type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. Insulin is also used along with glucose to treat hyperkalemia. Typically it is given by injection under the skin, but some forms may also be used by injection into a vein or muscle. There are various types of insulin, suitable for various time spans. The types are often all called insulin in the broad sense, although in a more precise sense, insulin is identical to the naturally occurring molecule whereas insulin analogues have slightly different molecules that allow for modified time of action. It is on the World Health Organization's List of Essential Medicines. In 2022, it was the 192nd most commonly prescribed medication in the United States, with more than 2 million prescriptions.

MiniMed Paradigm is a series of insulin pumps manufactured by Medtronic for patients with diabetes mellitus. The pump operates with a single AAA battery and uses a piston-plunger pump to infuse a programmed amount of insulin into the patient through a length of tubing. The Paradigm uses a one-way wireless radio frequency link to receive blood sugar measurements from select glucose meters. The Paradigm RT series adds the ability to receive data from a mated continuous blood-glucose monitor. Although the pump can use these measurements to assist in calculating a dose of insulin, no actual change in insulin delivery occurs without manual user-intervention.
Andrej Janež is a Slovenian diabetologist and diabetes researcher. Janež is the Head of Department of Endocrinology, Diabetes and Metabolic Disease at University Medical Centre Ljubljana, Assistant Professor for Internal Medicine at the Medical University Ljubljana, Chairman of the Advances in Diabetes and Insulin Therapy conference, member of the advisory board for peroral antidiabetic therapy in Servier Pharma, member for Slovenia in the Diabetes Education Study Group at European Association for the Study of Diabetes, and member of the European advisory board for continuous glucose monitoring system in development for Lifescan.
Tandem Diabetes Care, Inc. is an American medical device manufacturer based in San Diego, California. The company develops medical technologies for the treatment of diabetes and specifically insulin infusion therapy.
Bigfoot Biomedical Inc. is a medical technology start-up headquartered in Milpitas, California, founded by a team of people with personal connections to type 1 and type 2 diabetes.
Harry Keen CBE was an English diabetologist and a professor of human metabolism at Guy's Hospital. He was the first to identify microalbuminuria as a predictor of kidney disease in diabetics, and was an international authority on diabetes.
Rollin Turner "Woody" Woodyatt was an American physician, known for his contribution to the field of diabetes and other metabolic diseases. Woodyatt advocated a low-carbohydrate high-fat diet to treat diabetes.
















