The Institute of Cancer Research is a public research institute and a member institution of the University of London in London, United Kingdom, specialising in oncology. It was founded in 1909 as a research department of the Royal Marsden Hospital and joined the University of London in 2003. It has been responsible for a number of breakthrough discoveries, including that the basic cause of cancer is damage to DNA.
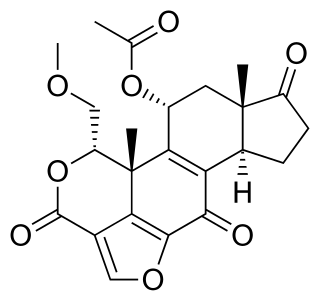
Wortmannin, a steroid metabolite of the fungi Penicillium funiculosum, Talaromyces wortmannii, is a non-specific, covalent inhibitor of phosphoinositide 3-kinases (PI3Ks). It has an in vitro inhibitory concentration (IC50) of around 5 nM, making it a more potent inhibitor than LY294002, another commonly used PI3K inhibitor. It displays a similar potency in vitro for the class I, II, and III PI3K members although it can also inhibit other PI3K-related enzymes such as mTOR, DNA-PKcs, some phosphatidylinositol 4-kinases, myosin light chain kinase (MLCK) and mitogen-activated protein kinase (MAPK) at high concentrations Wortmannin has also been reported to inhibit members of the polo-like kinase family with IC50 in the same range as for PI3K. The half-life of wortmannin in tissue culture is about 10 minutes due to the presence of the highly reactive C20 carbon that is also responsible for its ability to covalently inactivate PI3K. Wortmannin is a commonly used cell biology reagent that has been used previously in research to inhibit DNA repair, receptor-mediated endocytosis and cell proliferation.

The era of cancer chemotherapy began in the 1940s with the first use of nitrogen mustards and folic acid antagonist drugs. The targeted therapy revolution has arrived, but many of the principles and limitations of chemotherapy discovered by the early researchers still apply.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Cediranib is a potent inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases.
Synthetic lethality is defined as a type of genetic interaction where the combination of two genetic events results in cell death or death of an organism. Although the foregoing explanation is wider than this, it is common when referring to synthetic lethality to mean the situation arising by virtue of a combination of deficiencies of two or more genes leading to cell death, whereas a deficiency of only one of these genes does not. In a synthetic lethal genetic screen, it is necessary to begin with a mutation that does not result in cell death, although the effect of that mutation could result in a differing phenotype, and then systematically test other mutations at additional loci to determine which, in combination with the first mutation, causes cell death arising by way of deficiency or abolition of expression.

Olaparib, sold under the brand name Lynparza, is a medication for the maintenance treatment of BRCA-mutated advanced ovarian cancer in adults. It is a PARP inhibitor, inhibiting poly ADP ribose polymerase (PARP), an enzyme involved in DNA repair. It acts against cancers in people with hereditary BRCA1 or BRCA2 mutations, which include some ovarian, breast, and prostate cancers.

PARP inhibitors are a group of pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP).

Veliparib (ABT-888) is a potential anti-cancer drug acting as a PARP inhibitor. It kills cancer cells by blocking a protein called PARP, thereby preventing the repair of DNA or genetic damage in cancer cells and possibly making them more susceptible to anticancer treatments. Veliparib may make whole brain radiation treatment work more effectively against brain metastases from NSCLC. It has been shown to potentiate the effects of many chemotherapeutics, and as such has been part of many combination clinical trials.

Rucaparib, sold under the brand name Rubraca, is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It is approved in the United States and in Europe as third line treatment in BRCA-mutated ovarian cancer.

Hani Gabra PhD FRCPE FRCP is a British oncologist and Professor Emeritus in Medical Oncology at Imperial College London.
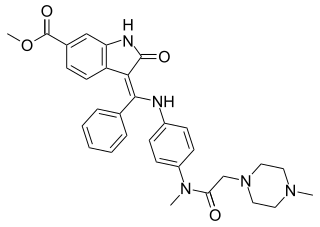
Nintedanib, sold under the brand names Ofev and Vargatef, is an oral medication used for the treatment of idiopathic pulmonary fibrosis and along with other medications for some types of non-small-cell lung cancer.
Angiokinase inhibitors are a new therapeutic target for the management of cancer. They inhibit tumour angiogenesis, one of the key processes leading to invasion and metastasis of solid tumours, by targeting receptor tyrosine kinases. Examples include nintedanib, afatinib and motesanib.
Alternating electric field therapy, sometimes called tumor treating fields (TTFields), is a type of electromagnetic field therapy using low-intensity, intermediate frequency electrical fields to treat cancer. A TTField-generating device manufactured by the Israeli company Novocure is approved in the United States and Europe for the treatment of newly diagnosed and recurrent glioblastoma multiforme (GBM), and is undergoing clinical trials for several other tumor types. Despite earning regulatory approval, the efficacy of this technology remains controversial among medical experts.

The Edinburgh Cancer Research Centre (ECRC), also known as the Cancer Research UK Edinburgh Centre and the University of Edinburgh Cancer Research Centre, is a center for basic, translational and clinical cancer research located in Edinburgh, Scotland. ECRC constitutes a part of the Institute of Genetics & Molecular Medicine (IGMM) and is positioned in direct proximity of the Western General Hospital, where most of its clinical activities take place.

Stephen Philip Jackson, FRS, FMedSci, is the Frederick James Quick Professor of Biology. He is a Senior Group Leader and Head of Cancer Research UK Laboratories at the Gurdon Institute.
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.
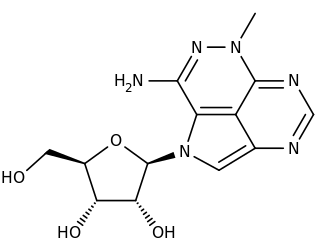
Triciribine is a cancer drug which was first synthesized in the 1970s and studied clinically in the 1980s and 1990s without success. Following the discovery in the early 2000s that the drug would be effective against tumours with hyperactivated Akt, it is now again under consideration in a variety of cancers. As PTX-200, the drug is currently in two early stage clinical trials in breast cancer and ovarian cancer being conducted by the small molecule drug development company Prescient Therapeutics.

Niraparib, sold under the brand name Zejula, is an anti-cancer medication used for the treatment of epithelial ovarian, fallopian tube, or primary peritoneal cancer. It is taken by mouth. It is a PARP inhibitor.

Berzosertib is a drug originally invented by Vertex Pharmaceuticals and licensed to Merck KGaA, Darmstadt, Germany for development. It acts as a potent inhibitor of the enzyme ataxia telangiectasia and Rad3 related (ATR) and with lower potency as an inhibitor of ATM serine/threonine kinase (ATM). These enzymes are both involved in detecting DNA damage as part of cell cycle checkpoints during cell division. By inhibiting their activity, berzosertib interferes with the ability of rapidly dividing cells to detect damage to DNA, and this makes it useful as a potential treatment for some forms of cancer by causing accumulation of DNA damage in the cancer cells and thus reducing their viability. It has progressed furthest in trials for the treatment of ovarian cancer, though also shows activity against numerous other cancer types.














