
Cloning is the process of producing individual organisms with identical genomes, either by natural or artificial means. In nature, some organisms produce clones through asexual reproduction; this reproduction of an organism by itself without a mate is known as parthenogenesis. In the field of biotechnology, cloning is the process of creating cloned organisms of cells and of DNA fragments.
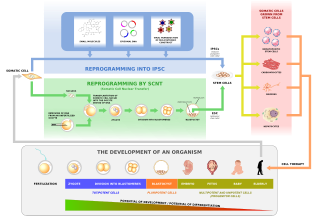
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissue. It does not refer to the natural conception and delivery of identical twins. The possibilities of human cloning have raised controversies. These ethical concerns have prompted several nations to pass laws regarding human cloning.

An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm cell. The resulting fusion of these two cells produces a single-celled zygote that undergoes many cell divisions that produce cells known as blastomeres. The blastomeres are arranged as a solid ball that when reaching a certain size, called a morula, takes in fluid to create a cavity called a blastocoel. The structure is then termed a blastula, or a blastocyst in mammals.

In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an denucleated oocyte and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal. In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques from foetal nuclei.

The Human Fertilisation and Embryology Authority (HFEA) is an executive non-departmental public body of the Department of Health and Social Care in the United Kingdom. It is a statutory body that regulates and inspects all clinics in the United Kingdom providing in vitro fertilisation (IVF), artificial insemination and the storage of human eggs, sperm or embryos. It also regulates human embryo research.

A designer baby is a baby whose genetic makeup has been selected or altered, often to exclude a particular gene or to remove genes associated with disease. This process usually involves analysing a wide range of human embryos to identify genes associated with particular diseases and characteristics, and selecting embryos that have the desired genetic makeup; a process known as preimplantation genetic diagnosis. Screening for single genes is commonly practiced, and polygenic screening is offered by a few companies. Other methods by which a baby's genetic information can be altered involve directly editing the genome before birth, which is not routinely performed and only one instance of this is known to have occurred as of 2019, where Chinese twins Lulu and Nana were edited as embryos, causing widespread criticism.
Christians take multiple positions in the debate on the morality of human cloning. Since Dolly the sheep was successfully cloned on 5 July 1996, and the possibility of cloning humans became a reality, Christian leaders have been pressed to take an ethical stance on its morality. While many Christians tend to disagree with the practice, such as Roman Catholics and a majority of fundamentalist pastors, including Southern Baptists, the views taken by various other Christian denominations are diverse and often conflicting. It is hard to pinpoint any one, definite stance of the Christian religion, since there are so many Christian denominations and so few official statements from each of them concerning the morality of human cloning.
The stem cell controversy is the consideration of the ethics of research involving the development and use of human embryos. Most commonly, this controversy focuses on embryonic stem cells. Not all stem cell research involves human embryos. For example, adult stem cells, amniotic stem cells, and induced pluripotent stem cells do not involve creating, using, or destroying human embryos, and thus are minimally, if at all, controversial. Many less controversial sources of acquiring stem cells include using cells from the umbilical cord, breast milk, and bone marrow, which are not pluripotent.
Stem cell research policy varies significantly throughout the world. There are overlapping jurisdictions of international organizations, nations, and states or provinces. Some government policies determine what is allowed versus prohibited, whereas others outline what research can be publicly financed. Of course, all practices not prohibited are implicitly permitted. Some organizations have issued recommended guidelines for how stem cell research is to be conducted.

The Human Fertilisation and Embryology Act 1990 is an Act of the Parliament of the United Kingdom. It created the Human Fertilisation and Embryology Authority which is in charge of human embryo research, along with monitoring and licensing fertility clinics in the United Kingdom.
Alan Osborne Trounson is an Australian embryologist with expertise in stem cell research. Trounson was the President of the California Institute for Regenerative Medicine between 2007 and 2014, a former Professor of Stem Cell Sciences and the Director of the Monash Immunology and Stem Cell Laboratories at Monash University, and retains the title of emeritus professor.
The Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, otherwise known as the European Convention on Bioethics or the European Bioethics Convention, is an international instrument aiming to prohibit the misuse of innovations in biomedicine and to protect human dignity. The Convention was opened for signature on 4 April 1997 in Oviedo, Spain and is thus otherwise known as the Oviedo Convention. The International treaty is a manifestation of the effort on the part of the Council of Europe to keep pace with developments in the field of biomedicine; it is notably the first multilateral binding instrument entirely devoted to biolaw. The Convention entered into force on 1 December 1999.
Stem cell laws are the law rules, and policy governance concerning the sources, research, and uses in treatment of stem cells in humans. These laws have been the source of much controversy and vary significantly by country. In the European Union, stem cell research using the human embryo is permitted in Sweden, Spain, Finland, Belgium, Greece, Britain, Denmark and the Netherlands; however, it is illegal in Germany, Austria, Ireland, Italy, and Portugal. The issue has similarly divided the United States, with several states enforcing a complete ban and others giving support. Elsewhere, Japan, India, Iran, Israel, South Korea, China, and Australia are supportive. However, New Zealand, most of Africa, and most of South America are restrictive.
A savior baby or savior sibling is a child who is conceived in order to provide a stem cell transplant to a sibling that is affected with a fatal disease, such as cancer or Fanconi anemia, that can best be treated by hematopoietic stem cell transplantation.
The multilateral foreign policy of the Holy See is particularly active on some issues, such as human rights, disarmament, and economic and social development, which are dealt with in international fora.
Mitochondrial replacement therapy (MRT), sometimes called mitochondrial donation, is the replacement of mitochondria in one or more cells to prevent or ameliorate disease. MRT originated as a special form of in vitro fertilisation in which some or all of the future baby's mitochondrial DNA (mtDNA) comes from a third party. This technique is used in cases when mothers carry genes for mitochondrial diseases. The therapy is approved for use in the United Kingdom. A second application is to use autologous mitochondria to replace mitochondria in damaged tissue to restore the tissue to a functional state. This has been used in clinical research in the United States to treat cardiac-compromised newborns.

The Human Reproductive Cloning Act 2001 was an Act of the Parliament of the United Kingdom "to prohibit the placing in a woman of a human embryo which has been created otherwise than by fertilisation". The act received Royal Assent on 4 December 2001.
Medical biology is a field of biology that has practical applications in medicine, health care and laboratory diagnostics. It includes many biomedical disciplines and areas of specialty that typically contains the "bio-" prefix such as:

The legal aspects of surrogacy in any particular jurisdiction tend to hinge on a few central questions:

Gábor Vajta is a medical doctor, human pathologist and mammalian embryologist living in Cairns, Queensland, Australia. Vajta was an Honorary Professor of the BGI College, Shenzhen, China, and Adjunct Professor of the Central Queensland University, Rockhampton, Queensland, Australia. After an early career in human pathology he turned to embryology in 1989 and obtained a Doctor of Science degree in Domestic Animal Embryology at the Royal Veterinary and Agricultural University in Copenhagen, Denmark in 1999. During the past 25 years he has co-developed several patents relating to embryology, most notably the method of Handmade Cloning (HMC), the Submarine Incubation System (SIS), the Open Pulled Straw (OPS) vitrification and the Well of the Well (WOW) system. Currently Professor Vajta is director of a consulting company providing services in human and domestic animal embryology all over the world, and founder and Chief Scientific Officer of VitaVitro Biotech Co., Ltd., Shenzhen, China.