Related Research Articles
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Avicine, tested and developed by AVI BioPharma, and also known as CTP-37 was trialled as a possible cancer vaccine to treat a number of different cancers. These included colorectal cancer, pancreatic cancer and prostate cancer. The treatment was trialled as and intended to be induced via intramuscular injection into the bloodstream, the location dependent on the treatment area.
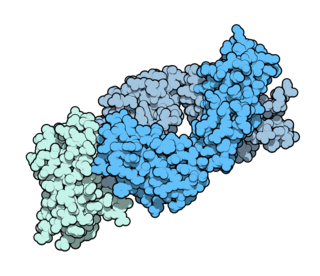
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
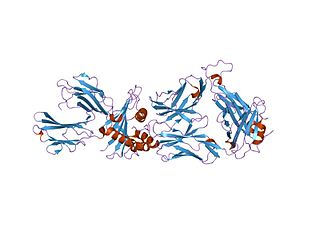
Cancer/testis antigen 1 also known as LAGE2 or LAGE2B is a protein that in humans is encoded by the CTAG1B gene. It is most often referenced by its alias NY-ESO-1.
Vaccine therapy is a type of treatment that uses a substance or group of substances to stimulate the immune system to destroy a tumor or infectious microorganisms such as bacteria or viruses.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy, T cells are extracted from the patient, genetically modified and cultured in vitro and returned to the same patient. Comparatively, allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.
Peptide-based synthetic vaccines are subunit vaccines made from peptides. The peptides mimic the epitopes of the antigen that triggers direct or potent immune responses. Peptide vaccines can not only induce protection against infectious pathogens and non-infectious diseases but also be utilized as therapeutic cancer vaccines, where peptides from tumor-associated antigens are used to induce an effective anti-tumor T-cell response.
Gustav Gaudernack is a scientist working in the development of cancer vaccines and cancer immunotherapy. He has developed various strategies in immunological treatment of cancer. He is involved in several ongoing cellular and immuno-gene therapeutic clinical trials and his research group has put major efforts into the development of various T cell-based immunotherapeutic strategies.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.
Racotumomab is a therapeutic cancer vaccine for the treatment of solid tumors that is currently under clinical development by ReComBio, an international public-private consortium with the participation of the Center of Molecular Immunology at Havana, Cuba (CIM) and researchers from Buenos Aires University and National University of Quilmes in Argentina. It induces the patient's immune system to generate a response against a cancer-specific molecular target with the purpose of blocking tumor growth, slowing disease progression and ultimately increasing patient survival.
ALECSAT technology is a novel method of epigenetic cancer immunotherapy being used by the company CytoVac. It uses a patient's own immune system to target tumor cells in prostate cancer, glioblastomas, and potentially pancreatic cancer. ALECSAT research, directed by Alexei Kirken and Karine Dzhandzhugazyan, has led to several clinical trials.

Immutep Ltd is a biotechnology company working primarily in the field of cancer immunotherapy using the LAG3 immune control mechanism. The company was originally built on CVac, a therapeutic cancer vaccine. In late 2014 the privately held French immunotherapy company Immutep SA was purchased by Prima Biotech.
Eftilagimod alpha is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings:

T lymphocytes are cells of the immune system that attack and destroy virus-infected cells, tumor cells and cells from transplanted organs. This occurs because each T cell is endowed with a highly specific receptor that can bind to an antigen present at the surface of another cell. The T cell receptor binds to a complex formed by a surface protein named "MHC" and a small peptide of about 9 amino-acids, which is located in a groove of the MHC molecule. This peptide can originate from a protein that remains within the cell. Whereas each T cell recognizes a single antigen, collectively the T cells are endowed with a large diversity of receptors targeted at a wide variety of antigens. T cells originate in the thymus. There a process named central tolerance eliminates the T cells that have a receptor recognizing an antigen present on normal cells of the organism. This enables the T cells to eliminate cells with "foreign" or "abnormal" antigens without harming the normal cells.
The immune-related response criteria (irRC) is a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment, where the compound being evaluated is an immuno-oncology drug. Immuno-oncology, part of the broader field of cancer immunotherapy, involves agents which harness the body's own immune system to fight cancer. Traditionally, patient responses to new cancer treatments have been evaluated using two sets of criteria, the WHO criteria and the response evaluation criteria in solid tumors (RECIST). The immune-related response criteria, first published in 2009, arose out of observations that immuno-oncology drugs would fail in clinical trials that measured responses using the WHO or RECIST Criteria, because these criteria could not account for the time gap in many patients between initial treatment and the apparent action of the immune system to reduce the tumor burden.
Cancer vaccine targeting CD4+ T cells is a type of vaccine used to treat existing cancer. Cancerous cells usually cannot be recognized by the human immune system, and therefore cannot be destroyed. Some researchers state that cancer can be treated by increasing the response of T cells, especially CD4+ T cells, to cancerous cells through cancer vaccine injection.

Cellular adoptive immunotherapy is a type of immunotherapy. Immune cells such as T-cells are usually isolated from patients for expansion or engineering purposes and reinfused back into patients to fight diseases using their own immune system. A major application of cellular adoptive therapy is cancer treatment, as the immune system plays a vital role in the development and growth of cancer. The primary types of cellular adoptive immunotherapies are T cell therapies. Other therapies include CAR-T therapy, CAR-NK therapy, macrophage-based immunotherapy and dendritic cell therapy.
Whole-cell vaccines are a type of vaccine that has been prepared in the laboratory from entire cells. Such vaccines simultaneously contain multiple antigens to activate the immune system. They induce antigen-specific T-cell responses.
References
- ↑ "Scancell Licensing Pact With US Health Agency". Wall Street Journal. 2010-05-11. Retrieved 2010-05-17.[ dead link ]