The plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein receptors organised in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains controversial. Indeed, Kervin and Overduin imply that lipid rafts are misconstrued protein islands, which they propose form through a proteolipid code. Nonetheless, it has been proposed that they are specialized membrane microdomains which compartmentalize cellular processes by serving as organising centers for the assembly of signaling molecules, allowing a closer interaction of protein receptors and their effectors to promote kinetically favorable interactions necessary for the signal transduction. Lipid rafts influence membrane fluidity and membrane protein trafficking, thereby regulating neurotransmission and receptor trafficking. Lipid rafts are more ordered and tightly packed than the surrounding bilayer, but float freely within the membrane bilayer. Although more common in the cell membrane, lipid rafts have also been reported in other parts of the cell, such as the Golgi apparatus and lysosomes.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
Lck is a 56 kDa protein that is found inside specialized cells of the immune system called lymphocytes. The Lck is a member of Src kinase family (SFK) and is important for the activation of T-cell receptor (TCR) signaling in both naive T cells and effector T cells. The role of Lck is less prominent in the activation or in the maintenance of memory CD8 T cells in comparison to CD4 T cells. In addition, the constitutive activity of the mouse Lck homolog varies among memory T cell subsets. It seems that in mice, in the effector memory T cell (TEM) population, more than 50% of Lck is present in a constitutively active conformation, whereas less than 20% of Lck is present as active form in central memory T cells. These differences are due to differential regulation by SH2 domain–containing phosphatase-1 (Shp-1) and C-terminal Src kinase.

ZAP-70 is a protein normally expressed near the surface membrane of lymphocytes. It is most prominently known to be recruited upon antigen binding to the T cell receptor (TCR), and it plays a critical role in T cell signaling.

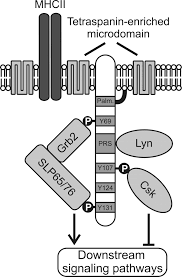
Lymphocyte cytosolic protein 2, also known as LCP2 or SLP-76, is a signal-transducing adaptor protein expressed in T cells and myeloid cells and is important in the signaling of T-cell receptors (TCRs). As an adaptor protein, SLP-76 does not have catalytic functions, primarily binding other signaling proteins to form larger signaling complexes. It is a key component of the signaling pathways of receptors with immunoreceptor tyrosine-based activation motifs (ITAMs) such as T-cell receptors, its precursors, and receptors for the Fc regions of certain antibodies. SLP-76 is expressed in T-cells and related lymphocytes like natural killer cells.

The Linker for activation of T cells, also known as linker of activated T cells or LAT, is a protein involved in the T-cell antigen receptor signal transduction pathway which in humans is encoded by the LAT gene. Alternative splicing results in multiple transcript variants encoding different isoforms.

GRB2-associated-binding protein 2 also known as GAB2 is a protein that in humans is encoded by the GAB2 gene.

Leukocyte surface antigen CD53 is a protein that in humans is encoded by the CD53 gene.

Proto-oncogene tyrosine-protein kinase Fyn is an enzyme that in humans is encoded by the FYN gene.
Tyrosine-protein kinase Lyn is a protein that in humans is encoded by the LYN gene.

Protein tyrosine phosphatase non-receptor type 22 (PTPN22) is a cytoplasmatic protein encoded by gene PTPN22 and a member of PEST family of protein tyrosine phosphatases. This protein is also called "PEST-domain Enriched Phosphatase" ("PEP") or "Lymphoid phosphatase" ("LYP"). The name LYP is used strictly for the human protein encoded by PTPN22, but the name PEP is used only for its mouse homolog. However, both proteins have similar biological functions and show 70% identity in amino acid sequence. PTPN22 functions as a negative regulator of T cell receptor (TCR) signaling, which maintains homeostasis of T cell compartment.

B-cell linker (BLNK) protein is expressed in B cells and macrophages and plays a large role in B cell receptor signaling. Like all adaptor proteins, BLNK has no known intrinsic enzymatic activity. Its function is to temporally and spatially coordinate and regulate downstream signaling effectors in B cell receptor (BCR) signaling, which is important in B cell development. Binding of these downstream effectors is dependent on BLNK phosphorylation. BLNK is encoded by the BLNK gene and is also known as SLP-65, BASH, and BCA.

Linker for activation of T-cells family member 2 is a protein that in humans is encoded by the LAT2 gene.

Dual adapter for phosphotyrosine and 3-phosphotyrosine and 3-phosphoinositide is a protein that in humans is encoded by the DAPP1 gene.

Lck-interacting transmembrane adapter 1 is a protein that in humans is encoded by the LIME1 gene.

Phosphoprotein associated with glycosphingolipid-enriched microdomains 1 is a protein that in humans is encoded by the PAG1 gene.
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell.
Kinetic-segregation is a model proposed for the mechanism of T-cell receptor (TCR) triggering. It offers an explanation for how TCR binding to its ligand triggers T-cell activation, based on size-sensitivity for the molecules involved. Simon J. Davis and Anton van der Merwe, University of Oxford, proposed this model in 1996. According to the model, TCR signalling is initiated by segregation of phosphatases with large extracellular domains from the TCR complex when binding to its ligand, allowing small kinases to phosphorylate intracellular domains of the TCR without inhibition. Its might also be applicable to other receptors of the Non-catalytic tyrosine-phosphorylated receptors family such as CD28.
Tyrosine-protein kinase CSK also known as C-terminal Src kinase is an enzyme that, in humans, is encoded by the CSK gene. This enzyme phosphorylates tyrosine residues located in the C-terminal end of Src-family kinases (SFKs) including SRC, HCK, FYN, LCK, LYN and YES1.
Non-catalytic tyrosine-phosphorylated receptors (NTRs), also called immunoreceptors or Src-family kinase-dependent receptors, are a group of cell surface receptors expressed by leukocytes that are important for cell migration and the recognition of abnormal cells or structures and the initiation of an immune response. These transmembrane receptors are not grouped into the NTR family based on sequence homology, but because they share a conserved signalling pathway utilizing the same signalling motifs. A signaling cascade is initiated when the receptors bind their respective ligand resulting in cell activation. For that tyrosine residues in the cytoplasmic tail of the receptors have to be phosphorylated, hence the receptors are referred to as tyrosine-phosphorylated receptors. They are called non-catalytic receptors, as the receptors have no intrinsic tyrosine kinase activity and cannot phosphorylate their own tyrosine residues. Phosphorylation is mediated by additionally recruited kinases. A prominent member of this receptor family is the T-cell receptor.