
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.
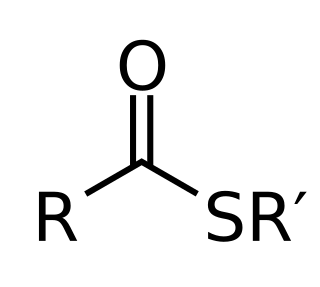
In chemistry thioesters are compounds with the functional group R–S–CO–R'. They are analogous to carboxylate esters with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester. They are the product of esterification between a carboxylic acid and a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.

Alamethicin is a channel-forming peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib. This residue strongly induces formation of alpha-helical structure. The peptide sequence is:
Native chemical ligation or NCL is an important extension of the chemical ligation field, a concept for constructing a large polypeptide formed by the assembly of two or more unprotected peptides segments. Especially, NCL is the most powerful ligation method for synthesizing native backbone proteins or modified proteins of moderate size.

Teicoplanin is an antibiotic used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis. It is a semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall synthesis.
Antimycins are produced as secondary metabolites by Streptomyces bacteria, a soil bacteria. These specialized metabolites likely function to kill neighboring organisms in order to provide the streptomyces bacteria with a competitive edge.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.

The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of E. coli. In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.
Chemical ligation is a set of techniques used for creating long peptide or protein chains. It is the second step of a convergent approach. First, smaller peptides containing 30-50 amino acids are prepared by conventional chemical peptide synthesis. Then, they are completely deprotected. Chemical ligation is the technique of coupling these peptides by chemoselective reaction to give a unique reaction product, usually in aqueous solution. With several coupling steps, proteins of up to 200-300 amino acids can be produced.
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.

S-Nitrosothiols, also known as thionitrites, are organic compounds or functional groups containing a nitroso group attached to the sulfur atom of a thiol. S-Nitrosothiols have the general formula RSNO, where R denotes an organic group. Originally suggested by Ignarro to serve as intermediates in the action of organic nitrates, endogenous S-nitrosothiols were discovered by Stamler and colleagues and shown to represent a main source of NO bioactivity in vivo. More recently, S-nitrosothiols have been implicated as primary mediators of protein S-nitrosylation, the oxidative modification of cysteine thiol that provides ubiquitous regulation of protein function.

The Liebeskind–Srogl coupling reaction is an organic reaction forming a new carbon–carbon bond from a thioester and a boronic acid using a metal catalyst. It is a cross-coupling reaction. This reaction was invented by and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. There are three generations of this reaction, with the first generation shown below. The original transformation used catalytic Pd(0), TFP = tris(2-furyl)phosphine as an additional ligand and stoichiometric CuTC = copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.

Racemic crystallography is a technique used in structural biology where crystals of a protein molecule are developed from the mixture of an ordinary chiral protein and its mirror image. These protein molecules consist of 'left-handed' L-amino acids that can be produced in bacteria, yeast or other cellular expression systems, whereas the mirror image of the protein molecules consist of 'right-handed' D-amino acids prepared by chemical synthesis. It has been used as a method to manufacture structures with high amounts of protein by increasing the rate of success in crystallizing proteins as well as eliminating unwanted phases in X-ray diffraction.
Thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a thione form (RC OH) and a thiol form (RC SH). These are sometimes also referred to as "carbothioic O-acid" and "carbothioic S-acid" respectively. Of these the thiol form is most common.
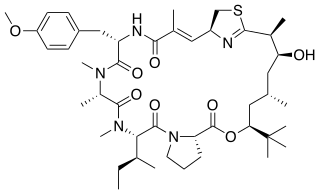
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.
3-hydroxydecanoyl-(acyl-carrier-protein) dehydratase (EC 4.2.1.60, D-3-hydroxydecanoyl-[acyl-carrier protein] dehydratase, 3-hydroxydecanoyl-acyl carrier protein dehydrase, 3-hydroxydecanoyl-acyl carrier protein dehydratase, beta-hydroxydecanoyl thioester dehydrase, beta-hydroxydecanoate dehydrase, beta-hydroxydecanoyl thiol ester dehydrase, FabA, beta-hydroxyacyl-acyl carrier protein dehydratase, HDDase, beta-hydroxyacyl-ACP dehydrase, (3R)-3-hydroxydecanoyl-[acyl-carrier-protein] hydro-lyase) is an enzyme with systematic name (3R)-3-hydroxydecanoyl-(acyl-carrier protein) hydro-lyase. This enzyme catalyses the following chemical reaction
Patrick L. Holland (born 1971) is the Conkey P. Whitehead Professor of Chemistry at Yale University. Holland's research focuses on low-coordinate and high-spin coordination complexes of iron and cobalt, that react with small molecules such as alkenes, arenes, and N2.












