Related Research Articles
Chemometrics is the science of extracting information from chemical systems by data-driven means. Chemometrics is inherently interdisciplinary, using methods frequently employed in core data-analytic disciplines such as multivariate statistics, applied mathematics, and computer science, in order to address problems in chemistry, biochemistry, medicine, biology and chemical engineering. In this way, it mirrors other interdisciplinary fields, such as psychometrics and econometrics.
In computational chemistry and molecular physics, Gaussian orbitals are functions used as atomic orbitals in the LCAO method for the representation of electron orbitals in molecules and numerous properties that depend on these.

In quantum mechanics, an excited state of a system is any quantum state of the system that has a higher energy than the ground state. Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation.

MOLPRO is a software package used for accurate ab initio quantum chemistry calculations. It is developed by Peter Knowles at Cardiff University and Hans-Joachim Werner at Universität Stuttgart in collaboration with other authors.
Oktay Sinanoğlu was a Turkish physical chemist and molecular biophysicist who made significant contributions to the theory of electron correlation in molecules, the statistical mechanics of clathrate hydrates, quantum chemistry, and the theory of solvation.
ZINDO is a semi-empirical quantum chemistry method used in computational chemistry. It is a development of the INDO method. It stands for Zerner's Intermediate Neglect of Differential Overlap, as it was developed by Michael Zerner and his coworkers in the 1970s. Unlike INDO, which was really restricted to organic molecules and those containing the atoms B to F, ZINDO covers a wide range of the periodic table, even including the rare-earth elements. There are two distinct versions of the method:
Jack David Dunitz FRS was a British chemist and widely known chemical crystallographer. He was Professor of Chemical Crystallography at the ETH Zurich from 1957 until his official retirement in 1990. He held Visiting Professorships in the United States, Israel, Japan, Canada, Spain and the United Kingdom.
Semi-empirical quantum chemistry methods are based on the Hartree–Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where the full Hartree–Fock method without the approximations is too expensive. The use of empirical parameters appears to allow some inclusion of electron correlation effects into the methods.

Theoretical Chemistry Accounts: Theory, Computation, and Modeling is a peer-reviewed scientific journal publishing original (primary) research and review articles in theoretical chemistry, physical chemistry, quantum chemistry, and computational chemistry. It was founded in 1962 as Theoretica Chimica Acta and was given its present name in 1998. The publisher is Springer Berlin Heidelberg. The impact factor of this journal is 2.233 (2014). The editor-in-chief is the team of Carlo Adamo and Ilaria Ciofini, the associate editor is Weitao Yang, and the chief advisory editor is Donald G. Truhlar.
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions.

Plumbane is an inorganic chemical compound with the chemical formula PbH4. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom. Derivatives of plumbane include lead tetrafluoride, PbF4, and tetraethyllead, (CH3CH2)4Pb.

Positronium hydride, or hydrogen positride is an exotic molecule consisting of a hydrogen atom bound to an exotic atom of positronium. Its formula is PsH. It was predicted to exist in 1951 by A Ore, and subsequently studied theoretically, but was not observed until 1990. R. Pareja, R. Gonzalez from Madrid trapped positronium in hydrogen laden magnesia crystals. The trap was prepared by Yok Chen from the Oak Ridge National Laboratory. In this experiment the positrons were thermalized so that they were not traveling at high speed, and they then reacted with H− ions in the crystal. In 1992 it was created in an experiment done by David M. Schrader and F.M. Jacobsen and others at the Aarhus University in Denmark. The researchers made the positronium hydride molecules by firing intense bursts of positrons into methane, which has the highest density of hydrogen atoms. Upon slowing down, the positrons were captured by ordinary electrons to form positronium atoms which then reacted with hydrogen atoms from the methane.

Hermann Hartmann was a German chemist and professor and researcher in physical and theoretical chemistry at the University of Frankfurt am Main. He contributed to all fields of physical chemistry and was instrumental in establishing theoretical chemistry by developing Ligand field theory (1947) and other quantum chemical models including the Hartmann Potential (1971). He also formulated a new perturbation theory (1970–1977) as part of his pioneering research towards a unified field theory of chemical bonding based on a non-linear Schrödinger equation (1980).

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Donald Gene Truhlar is an American scientist working in theoretical and computational chemistry and chemical physics with special emphases on quantum mechanics and chemical dynamics.
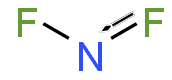
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer tetrafluorohydrazine.
Geerd Heinrich Friedrich Diercksen is a German theoretical chemist and a pioneer in computational chemistry. In 1963 he was awarded his PhD, supervised by Heinz-Werner Preuß at the Johann Wolfgang Goethe-Universität in Frankfurt am Main, in 1973 he was awarded his habilitation in Chemistry by the Technische Universität München and in 1983 he was appointed professor. From 1965 to 2001 he worked as scientific staff at the Max-Planck-Institut für Astrophysik and since 2001 he works there as scientist emeritus.
A nitrate nitrite, or nitrite nitrate, is a coordination complex or other chemical compound that contains both nitrite and nitrate anions (NO3− and NO2−). They are mixed-anion compounds, and they are mixed-valence compounds. Some have third anions. Many nitrite nitrate compounds are coordination complexes of cobalt. Such a substance was discovered by Wolcott Gibbs and Frederick Genth in 1857.

Scandium phosphide is an inorganic compound of scandium and phosphorus with the chemical formula ScP.
References
- ↑ D. N. Nanda and K. Jug, Theoretica Chimica Acta, 57, 95, (1980)
- ↑ K. Jug and D. N. Nanda, Theoretica Chimica Acta, 57, 107, (1980)
- ↑ K. Jug, R. Iffert and J. Schulz, International Journal of Quantum Chemistry, 32, 265, (1987)
- ↑ K. Jug and J. Schulz, Journal of Computational Chemistry, 9, 40, (1988)