
Gene therapy is a medical technology that aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.

Parvoviruses are a family of animal viruses that constitute the family Parvoviridae. They have linear, single-stranded DNA (ssDNA) genomes that typically contain two genes encoding for a replication initiator protein, called NS1, and the protein the viral capsid is made of. The coding portion of the genome is flanked by telomeres at each end that form into hairpin loops that are important during replication. Parvovirus virions are small compared to most viruses, at 23–28 nanometers in diameter, and contain the genome enclosed in an icosahedral capsid that has a rugged surface.

Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small replication-defective, nonenveloped viruses and have linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases (kb).

CD34 is a transmembrane phosphoglycoprotein protein encoded by the CD34 gene in humans, mice, rats and other species.
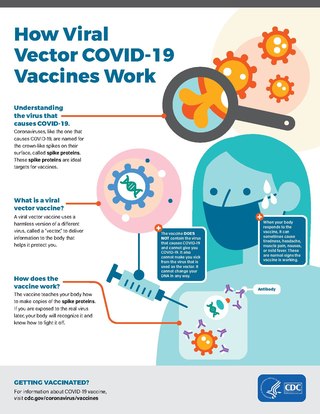
Viral vectors are modified viruses designed to deliver genetic material into cells. This process can be performed inside an organism or in cell culture. Viral vectors have widespread applications in basic research, agriculture, and medicine.
A helper dependent virus, also termed a gutless virus, is a synthetic viral vector dependent on the assistance of a helper virus in order to replicate, and can be used for purposes such as gene therapy. Naturally-occurring satellite viruses are also helper virus dependent, and can sometimes be modified to become viral vectors.

Gene delivery is the process of introducing foreign genetic material, such as DNA or RNA, into host cells. Gene delivery must reach the genome of the host cell to induce gene expression. Successful gene delivery requires the foreign gene delivery to remain stable within the host cell and can either integrate into the genome or replicate independently of it. This requires foreign DNA to be synthesized as part of a vector, which is designed to enter the desired host cell and deliver the transgene to that cell's genome. Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors.
Gene therapy using lentiviral vectors was being explored in early stage trials as of 2009.
Retinal gene therapy holds a promise in treating different forms of non-inherited and inherited blindness.
Isogenic human disease models are a family of cells that are selected or engineered to accurately model the genetics of a specific patient population, in vitro. They are provided with a genetically matched 'normal cell' to provide an isogenic system to research disease biology and novel therapeutic agents. They can be used to model any disease with a genetic foundation. Cancer is one such disease for which isogenic human disease models have been widely used.
Recombinant adeno-associated virus (rAAV) based genome engineering is a genome editing platform centered on the use of recombinant AAV vectors that enables insertion, deletion or substitution of DNA sequences into the genomes of live mammalian cells. The technique builds on Mario Capecchi and Oliver Smithies' Nobel Prize–winning discovery that homologous recombination (HR), a natural hi-fidelity DNA repair mechanism, can be harnessed to perform precise genome alterations in mice. rAAV mediated genome-editing improves the efficiency of this technique to permit genome engineering in any pre-established and differentiated human cell line, which, in contrast to mouse ES cells, have low rates of HR.

Alipogene tiparvovec, sold under the brand name Glybera, is a gene therapy treatment designed to reverse lipoprotein lipase deficiency (LPLD), a rare recessive disorder, due to mutations in LPL, which can cause severe pancreatitis. It was recommended for approval by the European Medicines Agency in July 2012, and approved by the European Commission in November of the same year. It was the first marketing authorisation for a gene therapy treatment in either the European Union or the United States.
Self-complementary adeno-associated virus (scAAV) is a viral vector engineered from the naturally occurring adeno-associated virus (AAV) to be used as a tool for gene therapy. Use of recombinant AAV (rAAV) has been successful in clinical trials addressing a variety of diseases. This lab-made progeny of rAAV is termed "self-complementary" because the coding region has been designed to form an intra-molecular double-stranded DNA template. A rate-limiting step for the standard AAV genome involves the second-strand synthesis since the typical AAV genome is a single-stranded DNA template. However, this is not the case for scAAV genomes. Upon infection, rather than waiting for cell mediated synthesis of the second strand, the two complementary halves of scAAV will associate to form one double stranded DNA (dsDNA) unit that is ready for immediate replication and transcription. The caveat of this construct is that instead of the full coding capacity found in rAAV (4.7–6kb) scAAV can only hold about half of that amount (≈2.4kb).

Lentiviral vectors in gene therapy is a method by which genes can be inserted, modified, or deleted in organisms using lentiviruses.
DNA-directed RNA interference (ddRNAi) is a gene-silencing technique that utilizes DNA constructs to activate a cell's endogenous RNA interference (RNAi) pathways. DNA constructs are designed to express self-complementary double-stranded RNAs, typically short-hairpin RNAs (shRNA), that bring about the silencing of a target gene or genes once processed. Any RNA, including endogenous messenger RNA (mRNAs) or viral RNAs, can be silenced by designing constructs to express double-stranded RNA complementary to the desired mRNA target.
James M. Wilson is an American biomedical researcher and CEO of two biotech companies, Gemma Biotherapeutics and Franklin Biolabs, focused on gene therapies. He previously served as the Director of the Gene Therapy Program, Rose H. Weiss Professor and Director of the Orphan Disease Center, and Professor of Medicine and Pediatrics at the Perelman School of Medicine at the University of Pennsylvania. Previously, he held the John Herr Musser endowed professorship at the Perelman School of Medicine.
Katherine A. High is an American doctor-scientist who is an emeritus professor at the Perelman School of Medicine at the University of Pennsylvania. She was the co-founder, president, and chief scientific officer of Spark Therapeutics and currently serves as President of Therapeutics at AskBio. She has worked in the area of gene therapy, performing both basic research and clinical investigations. She has been recognized for her distinguished contributions to the field, having designed, sponsored, and conducted the first clinical trial of an adeno-associated viral vector (AAV) gene therapy injected into the skeletal muscle (1999), the first trial of AAV gene therapy introduced into the liver (2001), and the first trial in the US of an AAV gene therapy injected into the subretinal space (2007).
Adeno-associated virus (AAV) has been researched as a viral vector in gene therapy for cancer treatment as an oncolytic virus. Currently there are not any FDA approved AAV cancer treatments, as the first FDA approved AAV treatment was approved December 2017. However, there are many Oncolytic AAV applications that are in development and have been researched.
Richard Jude Samulski is an American scientist, inventor, and academic recognized for his pioneering work in gene therapy and adeno-associated virus vectors (AAV) in the fields of molecular virology and pharmacology.
Mavis Agbandje-McKenna was a Nigerian-born British medical biophysicist, structural virologist, and a professor of structural biology, as well as the director of the Center for Structural Biology at the University of Florida in Gainesville, Florida. Agbandje-McKenna studied parvovirus structures using X-ray crystallography and cryogenic electron microscopy and did much of the initial work to elucidate the basic structure and function of adeno-associated viruses (AAVs). Her viral characterization and elucidation of antibody binding sites on AAV capsids has led to the development of viral capsid development and gene therapy approaches that evade immune detection and can be used to treat human diseases such as muscular dystrophies. Agbandje-McKenna was recognized with the 2020 American Society of Gene and Cell Therapy Outstanding Achievement Award for her contributions to the field. She died in 2021 from amyotrophic lateral sclerosis.







