Related Research Articles

Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested materials. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

Phagocytosis is the process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte.

Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae. Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery. Phagocytes occur in many species; some amoebae behave like macrophage phagocytes, which suggests that phagocytes appeared early in the evolution of life.
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns (PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A I, SR-A II, and MARCO.
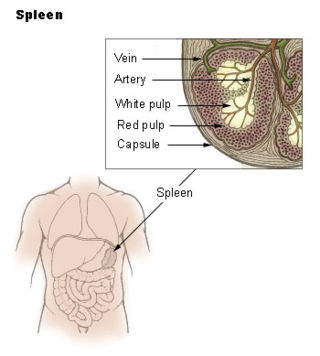
The marginal zone is the region at the interface between the non-lymphoid red pulp and the lymphoid white-pulp of the spleen.

Transcytosis is a type of transcellular transport in which various macromolecules are transported across the interior of a cell. Macromolecules are captured in vesicles on one side of the cell, drawn across the cell, and ejected on the other side. Examples of macromolecules transported include IgA, transferrin, and insulin. While transcytosis is most commonly observed in epithelial cells, the process is also present elsewhere. Blood capillaries are a well-known site for transcytosis, though it occurs in other cells, including neurons, osteoclasts and M cells of the intestine.

RAGE, also called AGER, is a 35 kilodalton transmembrane receptor of the immunoglobulin super family which was first characterized in 1992 by Neeper et al. Its name comes from its ability to bind advanced glycation endproducts (AGE), which include chiefly glycoproteins, the glycans of which have been modified non-enzymatically through the Maillard reaction. In view of its inflammatory function in innate immunity and its ability to detect a class of ligands through a common structural motif, RAGE is often referred to as a pattern recognition receptor. RAGE also has at least one other agonistic ligand: high mobility group protein B1 (HMGB1). HMGB1 is an intracellular DNA-binding protein important in chromatin remodeling which can be released by necrotic cells passively, and by active secretion from macrophages, natural killer cells, and dendritic cells.

Foam cells, also called lipid-laden macrophages, are a type of cell that contain cholesterol. These can form a plaque that can lead to atherosclerosis and trigger myocardial infarction and stroke.

A liver sinusoid is a type of capillary known as a sinusoidal capillary, discontinuous capillary or sinusoid, that is similar to a fenestrated capillary, having discontinuous endothelium that serves as a location for mixing of the oxygen-rich blood from the hepatic artery and the nutrient-rich blood from the portal vein.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein that in humans is encoded by the TFRC gene. TfR1 is required for iron import from transferrin into cells by endocytosis.

Stabilin-1 is a protein that in humans is encoded by the STAB1 gene.

Macrophage receptor with collagenous structure (MARCO) is a protein that in humans is encoded by the MARCO gene. MARCO is a class A scavenger receptor that is found on particular subsets of macrophages. Scavenger receptors are pattern recognition receptors (PRRs) found most commonly on immune cells. Their defining feature is that they bind to polyanions and modified forms of a type of cholesterol called low-density lipoprotein (LDL). MARCO is able to bind and phagocytose these ligands and pathogen-associated molecular patterns (PAMPs), leading to the clearance of pathogens and cell signaling events that lead to inflammation. As part of the innate immune system, MARCO clears, or scavenges, pathogens, which leads to inflammatory responses. The scavenger receptor cysteine-rich (SRCR) domain at the end of the extracellular side of MARCO binds ligands to activate the subsequent immune responses. MARCO expression on macrophages has been associated with tumor development and also with Alzheimer's disease, via decreased responses of cells when ligands bind to MARCO.

-Cytosis is a suffix that either refers to certain aspects of cells ie cellular process or phenomenon or sometimes refers to predominance of certain type of cells. It essentially means "of the cell". Sometimes it may be shortened to -osis and may be related to some of the processes ending with -esis or similar suffixes.
In anatomy the term reticuloendothelial system, often associated nowadays with the mononuclear phagocyte system (MPS), was employed by the beginning of the 20th century to denote a system of specialised cells that effectively clear colloidal vital stains from the blood circulation. The term is still used today, but its meaning has changed over the years, and is used inconsistently in present-day literature. Although RES is commonly associated exclusively with macrophages, recent research has revealed that the cells that accumulate intravenously administered vital stain belong to a highly specialised group of cells called scavenger endothelial cells (SECs), that are not macrophages.
Liver sinusoidal endothelial cells (LSECs) form the lining of the smallest blood vessels in the liver, also called the hepatic sinusoids. LSECs are highly specialized endothelial cells with characteristic morphology and function. They constitute an important part of the reticuloendothelial system (RES).
Endothelial cell tropism or endotheliotropism is a type of tissue tropism or host tropism that characterizes an pathogen's ability to recognize and infect an endothelial cell. Pathogens, such as viruses, can target a specific tissue type or multiple tissue types. Like other cells, the endothelial cell possesses several features that supports a productive viral infection a cell including, cell surface receptors, immune responses, and other virulence factors. Endothelial cells are found in various tissue types such as in the capillaries, veins, and arteries in the human body. As endothelial cells line these blood vessels and critical networks that extend access to various human organ systems, the virus entry into these cells can be detrimental to virus spread across the host system and affect clinical course of disease. Understanding the mechanisms of how viruses attach, enter, and control endothelial functions and host responses inform infectious disease understanding and medical countermeasures.
Apoptosis inhibitor of macrophage (AIM) is a protein produced by macrophages that regulates immune responses and inflammation. It plays a crucial role in key intracellular processes like lipid metabolism and apoptosis.
References
- ↑ Enomoto, K; Nishikawa, Y; Omori, Y; Tokairin, T; Yoshida, M; Ohi, N; Nishimura, T; Yamamoto, Y; Li, Q (December 2004). "Cell biology and pathology of liver sinusoidal endothelial cells". Medical Electron Microscopy. 37 (4): 208–15. doi:10.1007/s00795-004-0261-4. PMID 15614445. S2CID 8188662.
- ↑ Kamimoto, M; Rung-Ruangkijkrai, T; Iwanaga, T (June 2005). "Uptake ability of hepatic sinusoidal endothelial cells and enhancement by lipopolysaccharide". Biomedical Research (Tokyo, Japan). 26 (3): 99–107. doi: 10.2220/biomedres.26.99 . PMID 16011302.
- ↑ Wu, G; Li, Z (September 2009). "Glycoprotein clearance is rapid and suppressed by mannan in chicken embryos". Journal of Physiology and Biochemistry. 65 (3): 235–41. doi:10.1007/BF03180576. PMID 20119818. S2CID 30155614.
- ↑ Smedsrød, Bård; Seternes, Tore; Sørensen, Karen; Lindhe, Örjan; Sveinbjørnson, Baldur. Scavenger endothelial cells (Volume 7 ed.). Leiden, The Netherlands: Cells of the Hepatic Sinusoid. pp. 147–152.
- ↑ Sørensen, KK; Simon-Santamaria, J; McCuskey, RS; Smedsrød, B (20 September 2015). "Liver Sinusoidal Endothelial Cells". Comprehensive Physiology. 5 (4): 1751–74. doi: 10.1002/cphy.c140078 . PMID 26426467.
- ↑ Seternes, T; Sørensen, K; Smedsrød, B (28 May 2002). "Scavenger endothelial cells of vertebrates: a nonperipheral leukocyte system for high-capacity elimination of waste macromolecules". Proceedings of the National Academy of Sciences of the United States of America. 99 (11): 7594–7. Bibcode:2002PNAS...99.7594S. doi: 10.1073/pnas.102173299 . PMC 124295 . PMID 12032328.
- ↑ Campbell, F; Bos, FL; Sieber, S; Arias-Alpizar, G; Koch, BE; Huwyler, J; Kros, A; Bussmann, J (27 March 2018). "Directing Nanoparticle Biodistribution through Evasion and Exploitation of Stab2-Dependent Nanoparticle Uptake". ACS Nano. 12 (3): 2138–2150. doi:10.1021/acsnano.7b06995. PMC 5876619 . PMID 29320626.
- ↑ Verweij, FJ; Revenu, C; Arras, G; Dingli, F; Loew, D; Pegtel, DM; Follain, G; Allio, G; Goetz, JG; Zimmermann, P; Herbomel, P; Del Bene, F; Raposo, G; van Niel, G (25 February 2019). "Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo". Developmental Cell. 48 (4): 573–589.e4. doi: 10.1016/j.devcel.2019.01.004 . PMID 30745143.
- ↑ Das, D; Aradhya, R; Ashoka, D; Inamdar, M (1 May 2008). "Macromolecular uptake in Drosophila pericardial cells requires rudhira function". Experimental Cell Research. 314 (8): 1804–10. doi:10.1016/j.yexcr.2008.02.009. PMID 18355807.
- ↑ Palm, NB (1952). Storage and excretion of vital dyes in insects. With special regard to trypan blue (Almqvist Wiksell ed.). Stockholm: Arkiv für Zoologi. pp. 195–272.
- 1 2 3 Sørensen, KK; McCourt, P; Berg, T; Crossley, C; Le Couteur, D; Wake, K; Smedsrød, B (15 December 2012). "The scavenger endothelial cell: a new player in homeostasis and immunity". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 303 (12): R1217-30. doi:10.1152/ajpregu.00686.2011. PMID 23076875.
- ↑ Troha, Katia; Nagy, Peter; Pivovar, Andrew; Lazzaro, Brian P.; Hartley, Paul S.; Buchon, Nicolas (2019-09-16). "Nephrocytes Remove Microbiota-Derived Peptidoglycan from Systemic Circulation to Maintain Immune Homeostasis". Immunity. 51 (4): 625–637.e3. doi: 10.1016/j.immuni.2019.08.020 . ISSN 1097-4180. PMID 31564469.
- ↑ Rejman, J; Oberle, V; Zuhorn, IS; Hoekstra, D (1 January 2004). "Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis". The Biochemical Journal. 377 (Pt 1): 159–69. doi:10.1042/BJ20031253. PMC 1223843 . PMID 14505488.
- ↑ Seternes, T; Sørensen, K; Smedsrød, B (28 May 2002). "Scavenger endothelial cells of vertebrates: a nonperipheral leukocyte system for high-capacity elimination of waste macromolecules". Proceedings of the National Academy of Sciences of the United States of America. 99 (11): 7594–7. Bibcode:2002PNAS...99.7594S. doi: 10.1073/pnas.102173299 . PMC 124295 . PMID 12032328.
- ↑ Foroozandeh, P; Aziz, AA (25 October 2018). "Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles". Nanoscale Research Letters. 13 (1): 339. Bibcode:2018NRL....13..339F. doi: 10.1186/s11671-018-2728-6 . PMC 6202307 . PMID 30361809.