Related Research Articles

A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role.

Phagocytosis is the process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte.
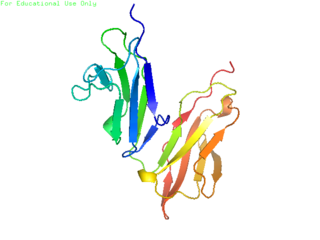
CD32, also known as FcγRII or FCGR2, is a surface receptor glycoprotein belonging to the Ig gene superfamily. CD32 can be found on the surface of a variety of immune cells. CD32 has a low-affinity for the Fc region of IgG antibodies in monomeric form, but high affinity for IgG immune complexes. CD32 has two major functions: cellular response regulation, and the uptake of immune complexes. Cellular responses regulated by CD32 include phagocytosis, cytokine stimulation, and endocytic transport. Dysregulated CD32 is associated with different forms of autoimmunity, including systemic lupus erythematosus. In humans, there are three major CD32 subtypes: CD32A, CD32B, and CD32C. While CD32A and CD32C are involved in activating cellular responses, CD32B is inhibitory.

A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass. These cells are involved in:

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns(PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A I, SR-A II, and MARCO.

The perisinusoidal space is a location in the liver between a hepatocyte and a sinusoid. It contains the blood plasma. Microvilli of hepatocytes extend into this space, allowing proteins and other plasma components from the sinusoids to be absorbed by the hepatocytes. Fenestration and discontinuity of the endothelium facilitates this transport. This space may be obliterated in liver disease, leading to decreased uptake by hepatocytes of nutrients and wastes such as bilirubin.

A liver sinusoid is a type of capillary known as a sinusoidal capillary, discontinuous capillary or sinusoid, that is similar to a fenestrated capillary, having discontinuous endothelium that serves as a location for mixing of the oxygen-rich blood from the hepatic artery and the nutrient-rich blood from the portal vein.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.

Low density lipoprotein receptor-related protein 1 (LRP1), also known as alpha-2-macroglobulin receptor (A2MR), apolipoprotein E receptor (APOER) or cluster of differentiation 91 (CD91), is a protein forming a receptor found in the plasma membrane of cells involved in receptor-mediated endocytosis. In humans, the LRP1 protein is encoded by the LRP1 gene. LRP1 is also a key signalling protein and, thus, involved in various biological processes, such as lipoprotein metabolism and cell motility, and diseases, such as neurodegenerative diseases, atherosclerosis, and cancer.

Stabilin-1 is a protein that in humans is encoded by the STAB1 gene.

Stabilin-2 is a protein that in humans is encoded by the STAB2 gene.

The liver is a major metabolic organ only found in vertebrate animals, which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it is located in the right upper quadrant of the abdomen, below the diaphragm and mostly shielded by the lower right rib cage. Its other metabolic roles include carbohydrate metabolism, the production of hormones, conversion and storage of nutrients such as glucose and glycogen, and the decomposition of red blood cells.
In anatomy the term "reticuloendothelial system", often associated nowadays with the mononuclear phagocyte system (MPS), was originally launched by the beginning of the 20th century to denote a system of specialised cells that effectively clear colloidal vital stains from the blood circulation. The term is still used today, but its meaning has changed over the years, and is used inconsistently in present-day literature. Although RES is commonly associated exclusively with macrophages, recent research has revealed that the cells that accumulate intravenously administered vital stain belong to a highly specialised group of cells called scavenger endothelial cells (SECs), that are not macrophages.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.
Liver cytology is the branch of cytology that studies the liver cells and its functions. The liver is a vital organ, in charge of almost all the body’s metabolism. Main liver cells are hepatocytes, Kupffer cells, and hepatic stellate cells; each one with a specific function.
Clark Lawrence Anderson is an internist and immunologist. He is professor emeritus in the Division of Immunology and Rheumatology, Department of Internal Medicine, Ohio State University (OSU), Columbus, Ohio, United States.
The term scavenger endothelial cell (SEC) was initially coined to describe a specialized sub-group of endothelial cells in vertebrates that express a remarkably high blood clearance activity. The term SEC has now been adopted by several scientists.
Endothelial cell tropism or endotheliotropism is a type of tissue tropism or host tropism that characterizes an pathogen's ability to recognize and infect an endothelial cell. Pathogens, such as viruses, can target a specific tissue type or multiple tissue types. Like other cells, the endothelial cell possesses several features that supports a productive viral infection a cell including, cell surface receptors, immune responses, and other virulence factors. Endothelial cells are found in various tissue types such as in the capillaries, veins, and arteries in the human body. As endothelial cells line these blood vessels and critical networks that extend access to various human organ systems, the virus entry into these cells can be detrimental to virus spread across the host system and affect clinical course of disease. Understanding the mechanisms of how viruses attach, enter, and control endothelial functions and host responses inform infectious disease understanding and medical countermeasures.
References
- ↑ Blouin, A; Bolender, RP; Weibel, ER (February 1977). "Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study". The Journal of Cell Biology. 72 (2): 441–55. doi:10.1083/jcb.72.2.441. PMC 2110997 . PMID 833203.
- ↑ Cogger, Victoria C.; Couteur, David G. Le (2009). "Fenestrations in the Liver Sinusoidal Endothelial Cell". The Liver. John Wiley & Sons, Ltd. pp. 389–406. doi:10.1002/9780470747919.ch27. ISBN 9780470747919. S2CID 82757901.
- ↑ Fraser, R; Cogger, VC; Dobbs, B; Jamieson, H; Warren, A; Hilmer, SN; Le Couteur, DG (April 2012). "The liver sieve and atherosclerosis". Pathology. 44 (3): 181–6. doi:10.1097/PAT.0b013e328351bcc8. PMID 22406487. S2CID 21014792.
- ↑ Wisse, E (March 1972). "An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells". Journal of Ultrastructure Research. 38 (5): 528–62. doi:10.1016/0022-5320(72)90089-5. PMID 4335119.
- ↑ Kjeken, R; Mousavi, SA; Brech, A; Gjøen, T; Berg, T (May 2001). "Fluid phase endocytosis of [125I]iodixanol in rat liver parenchymal, endothelial and Kupffer cells". Cell and Tissue Research. 304 (2): 221–30. doi:10.1007/s004410100348. PMID 11396716. S2CID 25938593.
- ↑ Sørensen, KK; McCourt, P; Berg, T; Crossley, C; Le Couteur, D; Wake, K; Smedsrød, B (15 December 2012). "The scavenger endothelial cell: a new player in homeostasis and immunity". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 303 (12): R1217-30. doi:10.1152/ajpregu.00686.2011. PMID 23076875.
- ↑ Sørensen, KK; Simon-Santamaria, J; McCuskey, RS; Smedsrød, B (20 September 2015). "Liver Sinusoidal Endothelial Cells". Comprehensive Physiology. 5 (4): 1751–74. doi: 10.1002/cphy.c140078 . PMID 26426467.
- ↑ Mousavi, SA; Sporstøl, M; Fladeby, C; Kjeken, R; Barois, N; Berg, T (September 2007). "Receptor-mediated endocytosis of immune complexes in rat liver sinusoidal endothelial cells is mediated by FcgammaRIIb2". Hepatology. 46 (3): 871–84. doi: 10.1002/hep.21748 . PMID 17680646.
- ↑ Pöhlmann, S; Soilleux, EJ; Baribaud, F; Leslie, GJ; Morris, LS; Trowsdale, J; Lee, B; Coleman, N; Doms, RW (27 February 2001). "DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans". Proceedings of the National Academy of Sciences of the United States of America. 98 (5): 2670–5. Bibcode:2001PNAS...98.2670P. doi: 10.1073/pnas.051631398 . PMC 30196 . PMID 11226297.
- ↑ Liu, W; Tang, L; Zhang, G; Wei, H; Cui, Y; Guo, L; Gou, Z; Chen, X; Jiang, D; Zhu, Y; Kang, G; He, F (30 April 2004). "Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node". The Journal of Biological Chemistry. 279 (18): 18748–58. doi: 10.1074/jbc.M311227200 . PMID 14711836.
- ↑ Martens, JH; Kzhyshkowska, J; Falkowski-Hansen, M; Schledzewski, K; Gratchev, A; Mansmann, U; Schmuttermaier, C; Dippel, E; Koenen, W; Riedel, F; Sankala, M; Tryggvason, K; Kobzik, L; Moldenhauer, G; Arnold, B; Goerdt, S (March 2006). "Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis". The Journal of Pathology. 208 (4): 574–89. doi:10.1002/path.1921. PMID 16440291. S2CID 84591741.
- ↑ Øie, CI; Appa, RS; Hilden, I; Petersen, HH; Gruhler, A; Smedsrød, B; Hansen, JB (December 2011). "Rat liver sinusoidal endothelial cells (LSECs) express functional low density lipoprotein receptor-related protein-1 (LRP-1)". Journal of Hepatology. 55 (6): 1346–52. doi:10.1016/j.jhep.2011.03.013. hdl: 10037/4078 . PMID 21703209.
- ↑ Boaru, SG; Borkham-Kamphorst, E; Tihaa, L; Haas, U; Weiskirchen, R (28 November 2012). "Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease". Journal of Inflammation. 9 (1): 49. doi: 10.1186/1476-9255-9-49 . PMC 3599703 . PMID 23192004.
- ↑ Knolle, PA; Wohlleber, D (May 2016). "Immunological functions of liver sinusoidal endothelial cells". Cellular & Molecular Immunology. 13 (3): 347–53. doi:10.1038/cmi.2016.5. PMC 4856811 . PMID 27041636.
- ↑ DeLeve, LD (May 2015). "Liver sinusoidal endothelial cells in hepatic fibrosis". Hepatology. 61 (5): 1740–6. doi:10.1002/hep.27376. PMC 4333127 . PMID 25131509.
- ↑ Xie, G; Wang, X; Wang, L; Wang, L; Atkinson, RD; Kanel, GC; Gaarde, WA; Deleve, LD (April 2012). "Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats". Gastroenterology. 142 (4): 918–927.e6. doi:10.1053/j.gastro.2011.12.017. PMC 3618963 . PMID 22178212.
- ↑ Naito, M; Wisse, E (10 July 1978). "Filtration effect of endothelial fenestrations on chylomicron transport in neonatal rat liver sinusoids". Cell and Tissue Research. 190 (3): 371–82. doi:10.1007/bf00219553. PMID 567529. S2CID 7431214.
- ↑ Fraser, R; Cogger, VC; Dobbs, B; Jamieson, H; Warren, A; Hilmer, SN; Le Couteur, DG (April 2012). "The liver sieve and atherosclerosis". Pathology. 44 (3): 181–6. doi:10.1097/PAT.0b013e328351bcc8. PMID 22406487. S2CID 21014792.
- ↑ Frank, MM; Lawley, TJ; Hamburger, MI; Brown, EJ (February 1983). "NIH Conference: Immunoglobulin G Fc receptor-mediated clearance in autoimmune diseases". Annals of Internal Medicine. 98 (2): 206–18. doi:10.7326/0003-4819-98-2-218. PMID 6824256.
- ↑ Ahmed, SS; Muro, H; Nishimura, M; Kosugi, I; Tsutsi, Y; Shirasawa, H (July 1995). "Fc receptors in liver sinusoidal endothelial cells in NZB/W F1 lupus mice: a histological analysis using soluble immunoglobulin G-immune complexes and a monoclonal antibody (2.4G2)". Hepatology. 22 (1): 316–24. doi:10.1002/hep.1840220143. hdl: 10271/1047 . PMID 7541388. S2CID 22425960.
- ↑ Hisazumi, J; Kobayashi, N; Nishikawa, M; Takakura, Y (July 2004). "Significant role of liver sinusoidal endothelial cells in hepatic uptake and degradation of naked plasmid DNA after intravenous injection". Pharmaceutical Research. 21 (7): 1223–8. doi:10.1023/B:PHAM.0000033009.17594.e5. PMID 15290863. S2CID 24013348.
- ↑ DeLeve, LD (November 2007). "Hepatic microvasculature in liver injury". Seminars in Liver Disease. 27 (4): 390–400. doi:10.1055/s-2007-991515. PMID 17979075.
- ↑ Godfrey, C; Desviat, LR; Smedsrød, B; Piétri-Rouxel, F; Denti, MA; Disterer, P; Lorain, S; Nogales-Gadea, G; Sardone, V; Anwar, R; El Andaloussi, S; Lehto, T; Khoo, B; Brolin, C; van Roon-Mom, WM; Goyenvalle, A; Aartsma-Rus, A; Arechavala-Gomeza, V (May 2017). "Delivery is key: lessons learnt from developing splice-switching antisense therapies". EMBO Molecular Medicine. 9 (5): 545–557. doi:10.15252/emmm.201607199. PMC 5412803 . PMID 28289078.
- ↑ DeLeve, LD (May 2013). "Liver sinusoidal endothelial cells and liver regeneration". The Journal of Clinical Investigation. 123 (5): 1861–6. doi:10.1172/JCI66025. PMC 3635729 . PMID 23635783.
- ↑ Aschoff, L. (1924). "Das reticulo-endotheliale System". Ergebnisse der Inneren Medizin und Kinderheilkunde (in German). Springer Berlin Heidelberg. pp. 1–118. doi:10.1007/978-3-642-90639-8_1. ISBN 978-3-642-88784-0.
{{cite book}}:|journal=ignored (help) - ↑ van Furth, R; Cohn, ZA; Hirsch, JG; Humphrey, JH; Spector, WG; Langevoort, HL (1972). "The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells". Bulletin of the World Health Organization. 46 (6): 845–52. PMC 2480884 . PMID 4538544.
- ↑ Wake, K; Kawai, Y; Smedsrød, B (2001). "Re-evaluation of the reticulo-endothelial system". Italian Journal of Anatomy and Embryology. 106 (2 Suppl 1): 261–9. PMID 11729964.
- ↑ Seternes, T; Sørensen, K; Smedsrød, B (28 May 2002). "Scavenger endothelial cells of vertebrates: a nonperipheral leukocyte system for high-capacity elimination of waste macromolecules". Proceedings of the National Academy of Sciences of the United States of America. 99 (11): 7594–7. Bibcode:2002PNAS...99.7594S. doi: 10.1073/pnas.102173299 . PMC 124295 . PMID 12032328.