Related Research Articles

Haematopoiesis is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells. In a healthy adult human, roughly ten billion to a hundred billion new blood cells are produced per day, in order to maintain steady state levels in the peripheral circulation.

The spleen is an organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter.

The lymphatic system, or lymphoid system, is an organ system in vertebrates that is part of the immune system and complementary to the circulatory system. It consists of a large network of lymphatic vessels, lymph nodes, lymphoid organs, lymphatic tissue and lymph. Lymph is a clear fluid carried by the lymphatic vessels back to the heart for re-circulation. The Latin word for lymph, lympha, refers to the deity of fresh water, "Lympha".

Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system. The mononuclear phagocytic system is part of the organism's immune system. The histiocyte is a tissue macrophage or a dendritic cell. Part of their job is to clear out neutrophils once they've reached the end of their lifespan.

Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives rise to biliverdin and iron. The body then traps the released iron and stores it as hemosiderin in tissues. Hemosiderin is also generated from the abnormal metabolic pathway of ferritin.

Extramedullary hematopoiesis refers to hematopoiesis occurring outside of the medulla of the bone. It can be physiologic or pathologic.
Malignant histiocytosis is a rare hereditary disease found in the Bernese Mountain Dog and humans, characterized by histiocytic infiltration of the lungs and lymph nodes. The liver, spleen, and central nervous system can also be affected. Histiocytes are a component of the immune system that proliferate abnormally in this disease. In addition to its importance in veterinary medicine, the condition is also important in human pathology.
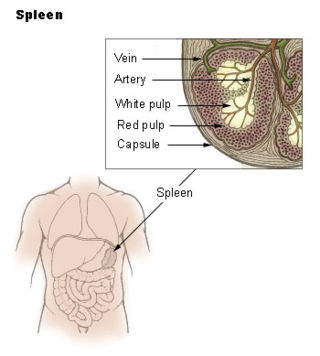
The red pulp of the spleen is composed of connective tissue known also as the cords of Billroth and many splenic sinusoids that are engorged with blood, giving it a red color. Its primary function is to filter the blood of antigens, microorganisms, and defective or worn-out red blood cells.
In anatomy and histology, the term wandering cell is used to describe cells that are found in connective tissue, but are not fixed in place. This term is used occasionally and usually refers to blood leukocytes in particular mononuclear phagocytes. Frequently, the term refers to circulating macrophages and has been used also for stationary macrophages fixed in tissues (histiocytes), which are sometimes referred to as "resting wandering cells".
A complement receptor is a membrane-bound receptor belonging to the complement system, which is part of the innate immune system. Complement receptors bind effector protein fragments that are produced in response to antigen-antibody complexes or damage-associated molecules. Complement receptor activation contributes to the regulation of inflammation, leukocyte extravasation, and phagocytosis; it also contributes to the adaptive immune response. Different complement receptors can participate in either the classical complement pathway, the alternative complement pathway, or both.

CD68 is a protein highly expressed by cells in the monocyte lineage, by circulating macrophages, and by tissue macrophages.

Epithelioid cells are derivatives of activated macrophages resembling epithelial cells.

White blood cells, also called immune cells or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. White blood cells are generally larger than red blood cells. They include three main subtypes: granulocytes, lymphocytes and monocytes.
In anatomy the term reticuloendothelial system, often associated nowadays with the mononuclear phagocyte system (MPS), was employed by the beginning of the 20th century to denote a system of specialised cells that effectively clear colloidal vital stains from the blood circulation. The term is still used today, but its meaning has changed over the years, and is used inconsistently in present-day literature. Although RES is commonly associated exclusively with macrophages, recent research has revealed that the cells that accumulate intravenously administered vital stain belong to a highly specialised group of cells called scavenger endothelial cells (SECs), that are not macrophages.
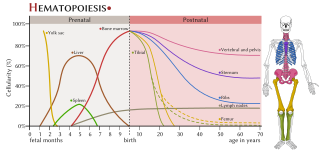
The haematopoietic system is the system in the body involved in the creation of the cells of blood.
Liver sinusoidal endothelial cells (LSECs) form the lining of the smallest blood vessels in the liver, also called the hepatic sinusoids. LSECs are highly specialized endothelial cells with characteristic morphology and function. They constitute an important part of the reticuloendothelial system (RES).

Gwendalyn J. Randolph is an American immunologist, the Emil R. Unanue Distinguished Professor in the Department of Immunology and Pathology at Washington University School of Medicine. During her postdoctoral work, Randolph characterized monocyte differentiation to dendritic cells and macrophages and made advances in our understanding of dendritic cell trafficking and the fate of monocytes recruited to sites of inflammation. Her lab has contributed to the Immunological Genome Project by characterizing macrophage gene expression and her laboratory has particularly contributed to our understanding of macrophages in the peritoneal cavity of mice and humans. Her work has also focused on the immunological mechanisms driving atherosclerosis and inflammatory bowel disease (IBD) by exploring lymphatic function and lipoprotein trafficking.
Dermal macrophages are macrophages in the skin that facilitate skin homeostasis by mediating wound repair, hair growth, and salt balance. Their functional role in these processes is the mediator of inflammation. They can acquire an M1 or M2 phenotype to promote or suppress an inflammatory response, thereby influencing other cells' activity via the production of pro-inflammatory or anti-inflammatory cytokines. Dermal macrophages' ability to acquire pro-inflammatory properties also potentiates them in cancer defence. M1 macrophages can suppress tumour growth in the skin by their pro-inflammatory properties. However, M2 macrophages support tumour growth and invasion by the production of Th2 cytokines such as TGFβ and IL-10. Thus, the exact contribution of each phenotype to cancer defence and the skin's homeostasis is still unclear.
References
- ↑ Mononuclear+Phagocyte+System at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Inderbir Singh (2006). Textbook of human histology. Jaypee Brothers Publishers. pp. 90–. ISBN 978-81-8061-809-3 . Retrieved 12 November 2010.[ permanent dead link ]
- ↑ Hume, David A (2006-02-01). "The mononuclear phagocyte system". Current Opinion in Immunology. Innate immunity / Antigen processing and recognition. 18 (1): 49–53. doi:10.1016/j.coi.2005.11.008. PMID 16338128.
- ↑ Lote, Christopher J. Principles of Renal Physiology, 5th edition. Springer. p. 37.