Haematopoiesis is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells. In a healthy adult human, roughly ten billion to a hundred billion new blood cells are produced per day, in order to maintain steady state levels in the peripheral circulation.

A dendritic cell (DC) is an antigen-presenting cell of the mammalian immune system. A DC's main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and adaptive immune systems.

Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae. Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery. Phagocytes occur in many species; some amoebae behave like macrophage phagocytes, which suggests that phagocytes appeared early in the evolution of life.

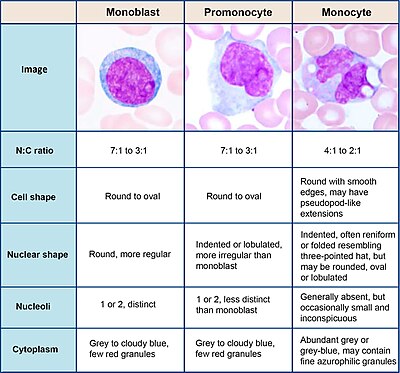
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also influence adaptive immune responses and exert tissue repair functions. There are at least three subclasses of monocytes in human blood based on their phenotypic receptors.

Erythropoiesis is the process which produces red blood cells (erythrocytes), which is the development from erythropoietic stem cell to mature red blood cell.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system. The mononuclear phagocytic system is part of the organism's immune system. The histiocyte is a tissue macrophage or a dendritic cell. Part of their job is to clear out neutrophils once they've reached the end of their lifespan.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".

A histiocytoma in the dog is a benign tumor. It is an abnormal growth in the skin of histiocytes (histiocytosis), a cell that is part of the immune system. A similar disease in humans, Hashimoto-Pritzker disease, is also a Langerhans cell histiocytosis. Dog breeds that may be more at risk for this tumor include Bulldogs, American Pit Bull Terriers, American Staffordshire Terriers, Scottish Terriers, Greyhounds, Boxers, and Boston Terriers. They also rarely occur in goats and cattle.

Interferon regulatory factors (IRF) are proteins which regulate transcription of interferons. Interferon regulatory factors contain a conserved N-terminal region of about 120 amino acids, which folds into a structure that binds specifically to the IRF-element (IRF-E) motifs, which is located upstream of the interferon genes. Some viruses have evolved defense mechanisms that regulate and interfere with IRF functions to escape the host immune system. For instance, the remaining parts of the interferon regulatory factor sequence vary depending on the precise function of the protein. The Kaposi sarcoma herpesvirus, KSHV, is a cancer virus that encodes four different IRF-like genes; including vIRF1, which is a transforming oncoprotein that inhibits type 1 interferon activity. In addition, the expression of IRF genes is under epigenetic regulation by promoter DNA methylation.

Granulopoiesis is a part of haematopoiesis, that leads to the production of granulocytes. A granulocyte, also referred to as a polymorphonuclear leukocyte (PMN), is a type of white blood cell that has multi lobed nuclei, usually containing three lobes, and has a significant amount of cytoplasmic granules within the cell. Granulopoiesis takes place in the bone marrow. It leads to the production of three types of mature granulocytes: neutrophils, eosinophils and basophils.
Lymphopoiesis (lĭm'fō-poi-ē'sĭs) is the generation of lymphocytes, one of the five types of white blood cells (WBCs). It is more formally known as lymphoid hematopoiesis.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.
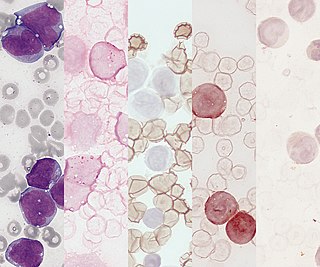
Acute monocytic leukemia is a type of acute myeloid leukemia. In AML-M5 >80% of the leukemic cells are of monocytic lineage. This cancer is characterized by a dominance of monocytes in the bone marrow. There is an overproduction of monocytes that the body does not need in the periphery. These overproduced monocytes interfere with normal immune cell production which causes many health complications for the affected individual.

Colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115, is a cell-surface protein encoded by the human CSF1R gene. CSF1R is a receptor that can be activated by two ligands: colony stimulating factor 1 (CSF-1) and interleukin-34 (IL-34). CSF1R is highly expressed in myeloid cells, and CSF1R signaling is necessary for the survival, proliferation, and differentiation of many myeloid cell types in vivo and in vitro. CSF1R signaling is involved in many diseases and is targeted in therapies for cancer, neurodegeneration, and inflammatory bone diseases.
In hematology, myelopoiesis in the broadest sense of the term is the production of bone marrow and of all cells that arise from it, namely, all blood cells. In a narrower sense, myelopoiesis also refers specifically to the regulated formation of myeloid leukocytes (myelocytes), including eosinophilic granulocytes, basophilic granulocytes, neutrophilic granulocytes, and monocytes.

White blood cells, also called immune cells, or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. White blood cells include three main subtypes; granulocytes, lymphocytes and monocytes.
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of immune cells from the myeloid lineage.
Dermal macrophages are macrophages in the skin that facilitate skin homeostasis by mediating wound repair, hair growth, and salt balance. Their functional role in these processes is the mediator of inflammation. They can acquire an M1 or M2 phenotype to promote or suppress an inflammatory response, thereby influencing other cells' activity via the production of pro-inflammatory or anti-inflammatory cytokines. Dermal macrophages' ability to acquire pro-inflammatory properties also potentiates them in cancer defence. M1 macrophages can suppress tumour growth in the skin by their pro-inflammatory properties. However, M2 macrophages support tumour growth and invasion by the production of Th2 cytokines such as TGFβ and IL-10. Thus, the exact contribution of each phenotype to cancer defence and the skin's homeostasis is still unclear.
Immune system contribution to regeneration of tissues generally involves specific cellular components, transcription of a wide variety of genes, morphogenesis, epithelia renewal and proliferation of damaged cell types. However, current knowledge reveals more and more studies about immune system influence that cannot be omitted. As the immune system exhibits inhibitory or inflammatory functions during regeneration, the therapies are focused on either stopping these processes or control the immune cells setting in a regenerative way, suggesting that interplay between damaged tissue and immune system response must be well-balanced. Recent studies provide evidence that immune components are required not only after body injury but also in homeostasis or senescent cells replacement.