
Haematopoiesis is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells. In a healthy adult human, roughly ten billion to a hundred billion new blood cells are produced per day, in order to maintain steady state levels in the peripheral circulation.

Red blood cells (RBCs), referred to as erythrocytes in academia and medical publishing, also known as red cells, erythroid cells, and rarely haematids, are the most common type of blood cell and the vertebrate's principal means of delivering oxygen to the body tissues—via blood flow through the circulatory system. Erythrocytes take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries.

A blood cell is a cell produced through hematopoiesis and found mainly in the blood. Major types of blood cells include red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes). Together, these three kinds of blood cells add up to a total 45% of the blood tissue by volume, with the remaining 55% of the volume composed of plasma, the liquid component of blood.

Anemia or anaemia is a blood disorder in which the blood has a reduced ability to carry oxygen. This can be due to a lower than normal number of red blood cells, a reduction in the amount of hemoglobin available for oxygen transport, or abnormalities in hemoglobin that impair its function.
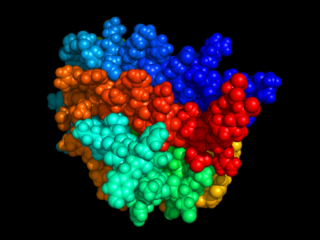
Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted in sufficient quantities to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease and mouth disease.

In hematology, reticulocytes are immature red blood cells (RBCs). In the process of erythropoiesis, reticulocytes develop and mature in the bone marrow and then circulate for about a day in the blood stream before developing into mature red blood cells. Like mature red blood cells, in mammals, reticulocytes do not have a cell nucleus. They are called reticulocytes because of a reticular (mesh-like) network of ribosomal RNA that becomes visible under a microscope with certain stains such as new methylene blue and Romanowsky stain.

Hereditary spherocytosis (HS) is a congenital hemolytic disorder wherein a genetic mutation coding for a structural membrane protein phenotype causes the red blood cells to be sphere-shaped (spherocytosis), rather than the normal biconcave disk shape. This abnormal shape interferes with the cells' ability to flex during blood circulation, and also makes them more prone to rupture under osmotic stress, mechanical stress, or both. Cells with the dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes and results in hemolytic anemia.
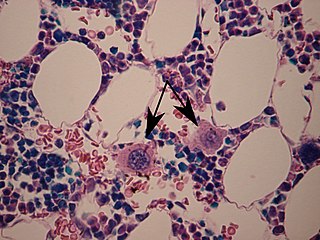
A megakaryocyte is a large bone marrow cell with a lobated nucleus that produces blood platelets (thrombocytes), which are necessary for normal clotting. In humans, megakaryocytes usually account for 1 out of 10,000 bone marrow cells, but can increase in number nearly 10-fold during the course of certain diseases. Owing to variations in combining forms and spelling, synonyms include megalokaryocyte and megacaryocyte.

Reticulocytosis is a laboratory finding in which the number of reticulocytes in the bloodstream is elevated. Reticulocytes account for approximately 0.5% to 2.5% of the total red blood cells in healthy adults and 2% to 6% in infants, but in reticulocytosis, this percentage rises. Reticulocytes are produced in the bone marrow and then released into the bloodstream, where they mature into fully developed red blood cells between 1-2 days. Reticulocytosis often reflects the body’s response to conditions rather than an independent disease process and can arise from a variety of causes such as blood loss or anemia.
Anemia of chronic disease (ACD) or anemia of chronic inflammation is a form of anemia seen in chronic infection, chronic immune activation, and malignancy. These conditions all produce elevation of interleukin-6, which stimulates hepcidin production and release from the liver. Hepcidin production and release shuts down ferroportin, a protein that controls export of iron from the gut and from iron storing cells. As a consequence, circulating iron levels are reduced. Other mechanisms may also play a role, such as reduced erythropoiesis. It is also known as anemia of inflammation, or anemia of inflammatory response.
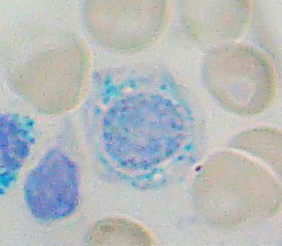
Sideroblastic anemia, or sideroachrestic anemia, is a form of anemia in which the bone marrow produces ringed sideroblasts rather than healthy red blood cells (erythrocytes). In sideroblastic anemia, the body has iron available but cannot incorporate it into hemoglobin, which red blood cells need in order to transport oxygen efficiently. The disorder may be caused either by a genetic disorder or indirectly as part of myelodysplastic syndrome, which can develop into hematological malignancies.

A proerythroblast is a precursor cell to the normoblast, as the earliest of four stages in its development.
Reticulocytopenia is the medical term for an abnormal decrease in circulating red blood cell precursors (reticulocytes) that can lead to anemia due to resulting low red blood cell (erythrocyte) production. Reticulocytopenia may be an isolated finding or it may not be associated with abnormalities in other hematopoietic cell lineages such as those that produce white blood cells (leukocytes) or platelets (thrombocytes), a decrease in all three of these lineages is referred to as pancytopenia.

The erythropoietin receptor (EpoR) is a protein that in humans is encoded by the EPOR gene. EpoR is a 52 kDa peptide with a single carbohydrate chain resulting in an approximately 56–57 kDa protein found on the surface of EPO responding cells. It is a member of the cytokine receptor family. EpoR pre-exists as dimers. These dimers were originally thought to be formed by extracellular domain interactions, however, it is now assumed that it is formed by interactions of the transmembrane domain and that the original structure of the extracellular interaction site was due to crystallisation conditions and does not depict the native conformation. Binding of a 30 kDa ligand erythropoietin (Epo), changes the receptor's conformational change, resulting in the autophosphorylation of Jak2 kinases that are pre-associated with the receptor. At present, the best-established function of EpoR is to promote proliferation and rescue of erythroid progenitors from apoptosis.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.

CFU-GEMM is a colony forming unit that generates myeloid cells. CFU-GEMM cells are the oligopotential progenitor cells for myeloid cells; they are thus also called common myeloid progenitor cells or myeloid stem cells. "GEMM" stands for granulocyte, erythrocyte, monocyte, megakaryocyte.

The term CFU-E denotes a hematopoietic stem cell in the bone marrow which eventually matures into a red blood cell. It arises from CFU-GEMM and gives rise to proerythroblasts.
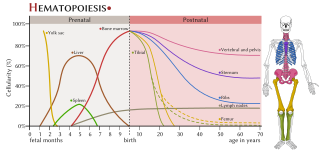
The haematopoietic system is the system in the body involved in the creation of the cells of blood.

A nucleated red blood cell (NRBC), also known by several other names, is a red blood cell that contains a cell nucleus. Almost all vertebrate organisms have hemoglobin-containing cells in their blood, and with the exception of mammals, all of these red blood cells are nucleated. In mammals, NRBCs occur in normal development as precursors to mature red blood cells in erythropoiesis, the process by which the body produces red blood cells.

Erythroferrone is a protein hormone encoded in humans by the ERFE gene. Erythroferrone is produced by erythroblasts, inhibits the production of hepcidin in the liver, and so increases the amount of iron available for hemoglobin synthesis. Skeletal muscle secreted ERFE has been shown to maintain systemic metabolic homeostasis.
















