
Chemokines are a family of small cytokines, or signaling proteins secreted by cells. Their name is derived from their ability to induce directed chemotaxis in nearby responsive cells; they are chemotactic cytokines.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

Fas ligand is a type-II transmembrane protein that belongs to the tumor necrosis factor (TNF) family. Its binding with its receptor induces apoptosis. Fas ligand/receptor interactions play an important role in the regulation of the immune system and the progression of cancer.

A Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.
A co-receptor is a cell surface receptor that binds a signalling molecule in addition to a primary receptor in order to facilitate ligand recognition and initiate biological processes, such as entry of a pathogen into a host cell.

Endoglin (ENG) is a type I membrane glycoprotein located on cell surfaces and is part of the TGF beta receptor complex. It is also commonly referred to as CD105, END, FLJ41744, HHT1, ORW and ORW1. It has a crucial role in angiogenesis, therefore, making it an important protein for tumor growth, survival and metastasis of cancer cells to other locations in the body.
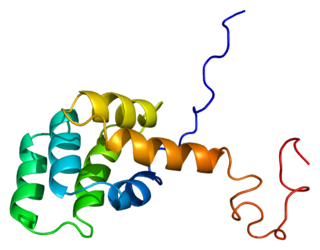
The Fas receptor, also known as Fas, FasR, apoptosis antigen 1, cluster of differentiation 95 (CD95) or tumor necrosis factor receptor superfamily member 6 (TNFRSF6), is a protein that in humans is encoded by the FAS gene. Fas was first identified using a monoclonal antibody generated by immunizing mice with the FS-7 cell line. Thus, the name Fas is derived from FS-7-associated surface antigen.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

Death receptor 4 (DR4), also known as TRAIL receptor 1 (TRAILR1) and tumor necrosis factor receptor superfamily member 10A (TNFRSF10A), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis.

Signal regulatory protein α (SIRPα) is a regulatory membrane glycoprotein from SIRP family expressed mainly by myeloid cells and also by stem cells or neurons.

LIGHT, also known as tumor necrosis factor superfamily member 14 (TNFSF14), is a secreted protein of the TNF superfamily. It is recognized by herpesvirus entry mediator (HVEM), as well as decoy receptor 3.

Herpesvirus entry mediator (HVEM), also known as tumor necrosis factor receptor superfamily member 14 (TNFRSF14), is a human cell surface receptor of the TNF-receptor superfamily.

Macrophage scavenger receptor 1, also known as MSR1, is a protein which in humans is encoded by the MSR1 gene. MSR1 has also been designated CD204.

Galectin-3-binding protein is a protein that in humans is encoded by the LGALS3BP gene.

CD6 is a human protein encoded by the CD6 gene.

Macrophage receptor MARCO also known as macrophage receptor with collagenous structure (MARCO) is a protein that in humans is encoded by the MARCO gene. MARCO is a class A scavenger receptor that is found on particular subsets of macrophages. Scavenger receptors are pattern recognition receptors (PRRs) and are most commonly found on immune cells. Their defining feature is that they bind to polyanions and modified forms of a type of cholesterol called low-density lipoprotein (LDL). MARCO is able to bind and phagocytose these ligands and pathogen-associated molecular patterns (PAMPs), leading to the clearance of pathogens as well as causing downstream effects in the cell that lead to inflammation. As part of the innate immune system, MARCO clears, or scavenges, pathogens and leads to inflammatory responses. The scavenger receptor cysteine-rich (SRCR) domain at the end of the extracellular side of MARCO is responsible for ligand binding and the subsequent immune responses. MARCO expression on macrophages is also associated with diseases since Alzheimer's disease is associated with decreased response within the cell when a ligand binds to MARCO.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.

The LU domain is an evolutionally conserved protein domain of the three-finger protein superfamily. This domain is found in the extracellular domains of cell-surface receptors and in either GPI-anchored or secreted globular proteins, for example the Ly-6 family, CD59, and Sgp-2.
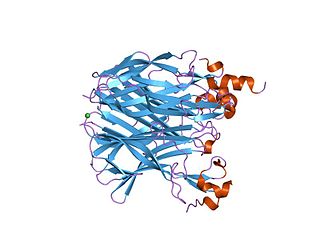
In molecular biology, this protein domain, TACI-CRD2 represents the second cysteine-rich protein domain found in the TACI family of proteins. Members of this family are predominantly found in tumour necrosis factor receptor superfamily, member 13b (TACI), and are required for binding to the ligands APRIL and BAFF. TACI-CRD2 stands for Transmembrane Activator and CAML Interactor- Cysteine Rich Domain 2.

Scavenger receptor cysteine rich family member with 4 domains is a protein that in humans is encoded by the SSC4D gene.
















