Related Research Articles

Oligodendrogliomas are a type of glioma that are believed to originate from the oligodendrocytes of the brain or from a glial precursor cell. They occur primarily in adults but are also found in children.

An ependymoma is a tumor that arises from the ependyma, a tissue of the central nervous system. Usually, in pediatric cases the location is intracranial, while in adults it is spinal. The common location of intracranial ependymomas is the floor of the fourth ventricle. Rarely, ependymomas can occur in the pelvic cavity.

A blastoma is a type of cancer, more common in children, that is caused by malignancies in precursor cells, often called blasts. Examples are nephroblastoma, medulloblastoma, and retinoblastoma. The suffix -blastoma is used to imply a tumor of primitive, incompletely differentiated cells, e.g., chondroblastoma is composed of cells resembling the precursor of chondrocytes.
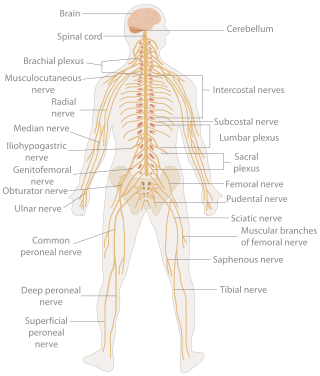
A neurological examination is the assessment of sensory neuron and motor responses, especially reflexes, to determine whether the nervous system is impaired. This typically includes a physical examination and a review of the patient's medical history, but not deeper investigation such as neuroimaging. It can be used both as a screening tool and as an investigative tool, the former of which when examining the patient when there is no expected neurological deficit and the latter of which when examining a patient where you do expect to find abnormalities. If a problem is found either in an investigative or screening process, then further tests can be carried out to focus on a particular aspect of the nervous system.

Medulloblastoma is a common type of primary brain cancer in children. It originates in the part of the brain that is towards the back and the bottom, on the floor of the skull, in the cerebellum, or posterior fossa.

Hemangioblastomas, or haemangioblastomas, are vascular tumors of the central nervous system that originate from the vascular system, usually during middle age. Sometimes, these tumors occur in other sites such as the spinal cord and retina. They may be associated with other diseases such as polycythemia, pancreatic cysts and Von Hippel–Lindau syndrome. Hemangioblastomas are most commonly composed of stromal cells in small blood vessels and usually occur in the cerebellum, brainstem or spinal cord. They are classed as grade I tumors under the World Health Organization's classification system.

An atypical teratoid rhabdoid tumor (AT/RT) is a rare tumor usually diagnosed in childhood. Although usually a brain tumor, AT/RT can occur anywhere in the central nervous system (CNS), including the spinal cord. About 60% will be in the posterior cranial fossa. One review estimated 52% in the posterior fossa, 39% are supratentorial primitive neuroectodermal tumors (sPNET), 5% are in the pineal, 2% are spinal, and 2% are multifocal.
Todd R. Golub is a professor of pediatrics at the Harvard Medical School, the Charles A. Dana Investigator in Human Cancer Genetics at the Dana–Farber Cancer Institute, and the Director and a founding member of the Broad Institute of MIT and Harvard.
Choroid plexus tumors are a rare type of cancer that occur from the brain tissue called choroid plexus of the brain. Choroid plexus tumors are uncommon tumors of the central nervous system that account for 0.5–0.6% of intracranial neoplasms in people of all ages. Choroid plexus papilloma, atypical choroid plexus papilloma, and choroid plexus carcinoma are the three World Health Organization types for these neoplasms. Children under the age of five account for 10% of cases of choroid plexus tumors. In children and adults, respectively, the lateral ventricle and the fourth ventricle are common locations, About 5% of all choroid plexus tumors are located in the third ventricle. Along with other unusual places such the cerebellopontine angle, the Luschka foramen, or brain parenchyma, the third ventricle is a rare location for choroid plexus tumors. Together, atypical choroid plexus papilloma, and choroid plexus carcinoma make up around 25% of all choroid plexus tumors. Although there have been reports of third ventricle choroid plexus papillomas in people in their fifth decade of life, only 14% of choroid plexus tumors are reported to arise in infants. Most findings indicate that choroid plexus tumors have no sex predilection.

Protein patched homolog 1 is a protein that is the member of the patched family and in humans is encoded by the PTCH1 gene.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).
Pediatric ependymomas are similar in nature to the adult form of ependymoma in that they are thought to arise from radial glial cells lining the ventricular system. However, they differ from adult ependymomas in which genes and chromosomes are most often affected, the region of the brain they are most frequently found in, and the prognosis of the patients. Children with certain hereditary diseases, such as neurofibromatosis type II (NF2), have been found to be more frequently afflicted with this class of tumors, but a firm genetic link remains to be established. Symptoms associated with the development of pediatric ependymomas are varied, much like symptoms for a number of other pediatric brain tumors including vomiting, headache, irritability, lethargy, and changes in gait. Although younger children and children with invasive tumor types generally experience less favorable outcomes, total removal of the tumors is the most conspicuous prognostic factor for both survival and relapse.
The Collaborative Ependymoma Research Network (CERN) Foundation is a nonprofit organization composed of scientists and adult and pediatric cancer researchers who work together to develop new treatments for ependymoma, a type of primary brain or spinal cord tumor that occurs in both children and adults, and improve the outcomes and care of patients. The organization is headquartered in Dayton, Ohio, USA.

MYB proto-oncogene like 1 is a protein that in humans is encoded by the MYBL1 gene.

MicroRNA 517c is a microRNA that in humans is encoded by the MIR517C gene.

Professor Richard James Gilbertson is a Senior Group Leader at the Cancer Research UK Cambridge Institute, University of Cambridge. He is the Li Ka Shing Chair of Oncology, and Director of the CRUK Cambridge Major Centre and the Children's Brain Tumour Centre of Excellence.
Tina Young Poussaint is a professor of radiology at the Harvard Medical School and a Neuroradiologist at the Boston Children's Hospital. In 2010 she served as President of the American Society of Pediatric Neuroradiology.
Embryonal tumor with multilayered rosettes (ETMR) is an embryonal central nervous system tumor. It is considered an embryonal tumor because it arises from cells partially differentiated or still undifferentiated from birth, usually neuroepithelial cells, stem cells destined to turn into glia or neurons. It can occur anywhere within the brain and can have multiple sites of origins, with a high probability of metastasis through cerebrospinal fluid (CSF). Metastases outside the central nervous system have been reported, but remain rare.

Soma Sengupta is a British-American physician-scientist. She is a specialty board certified neuro-oncologist board certified Neurologist and fellowship-trained in Integrative Medicine. Her clinical interests span treatment of brain tumor patients, integrative approaches in neurology and oncology, as well as healthcare policy. She is a full-time faculty member in the Departments of Neurology and Neurosurgery at the University of North Carolina at Chapel Hill, where she is a Full Professor, Vice Chair, member of the Lineberger Comprehensive Cancer Center, and Chief of the Division of Neuro-Oncology. She is also a Bye Fellow at Lucy Cavendish College, University of Cambridge, U.K.
Diffuse leptomeningeal glioneuronal tumor (DLGNT) is a rare, primary CNS tumor, classified as distinct entity in 2016 and described as diffuse oligodendroglial-like leptomeningeal tumor of children in the 2016 classification of CNS neoplasms by the WHO., Typically, it's considered juvenile tumors but can occur in adults, the average age of diagnosis is five years. It's characterised by wide leptomeningeal spread with male predominance, like histopathology of neurocytoma, oligodendrocyte-like cytopathology, bland appearance, and severe clinical behaviour. Children's basal cisterns and inter-hemispheric fissures are typically involved in plaque like subarachnoid tumors. A common related intraparenchymal lesion is a spinal lesion. However, in certain situations, superficial parenchyma or Virchow-Robin gaps were affected.
Molecular and genetic investigations frequently show a combination of KIAA1549 and the serine/threonine protein kinase BRAF gene, and also deletions of the short arm of chromosome number 1 and/or the long arm of chromosome number 19.
References
- 1 2 Confronting Cancer: Telling the Threatening Tumors from the Harmless Ones. NY Times April 9, 2002.
- 1 2 3 Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons W, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol 2012; 123:456-472.
- 1 2 3 Louis DN, et al. WHO Classification of Tumours of the Central Nervous System, 2016.
- ↑ Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med 1990; 323:1651-1657.
- ↑ Lipton J, Megerian JT, Kothare SV, Cho YJ, Shanahan T, Chart H, Ferber R, Adler-Golden L, Cohen LE, Czeisler CA, Pomeroy SL. Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology 2009; 73:323-25.
- ↑ Jones HR, Burns TM, Aminoff MJ, Pomeroy SL (eds). The Netter Collection of Medical Illustrations: Nervous System. (2nd edition). Philadelphia: Elsevier 2013.
- ↑ Daroff RB, Jankovic J, Mazziotta JC, Pomeroy SL (eds). Bradley's Neurology in Clinical Practice (7th edition). Philadelphia: Elsevier 2016.