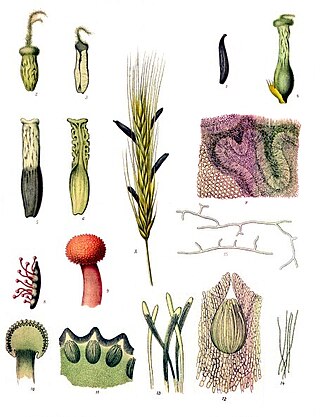
Ergot or ergot fungi refers to a group of fungi of the genus Claviceps.
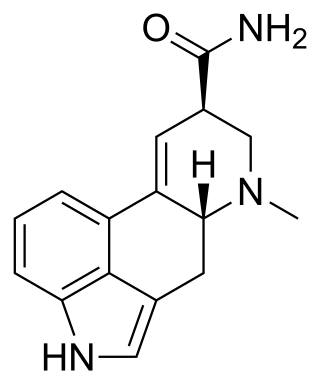
Ergine, also known as d-lysergic acid amide (LSA) and d-lysergamide, is an ergoline alkaloid that occurs in various species of vines of the Convolvulaceae and some species of fungi. The psychedelic properties in the seeds of ololiuhqui, Hawaiian baby woodrose and morning glories have been linked to ergine and/or isoergine, its epimer, as it is an alkaloid present in the seeds.
A mycotoxin is a toxic secondary metabolite produced by fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.
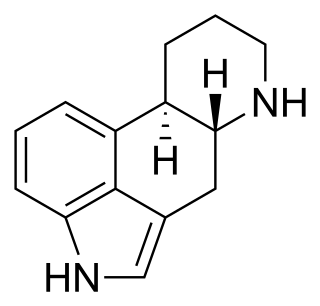
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
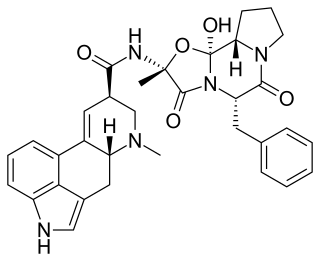
Ergotamine, sold under the brand name Ergomar among others, is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It is structurally similar to several neurotransmitters, and it acts as a vasoconstrictor. It is used for acute migraines, sometimes with caffeine as the combination ergotamine/caffeine.

D-Lysergic acid α-hydroxyethylamide, also known as D-lysergic acid methyl carbinolamide, is a Lysergamide and alkaloid of the Ergoline family, it is present in higher concentrations in the parasitic fungi species "Claviceps", mainly the Claviceps paspali, also in Claviceps Purpurea. This fungi grows in various species in the Convolvulaceae family like the Ipomoea violacea, the Rivea corymbosa (Ololiuhqui), and the Argyreia nervosa. Heavenly Blue Morning Glory and Hawaiian Baby Woodrose especially contain high amounts of LSH, with content varying between species and by how fresh the seeds are. LSH is a psychoactive Ergoline and has effects similar to LSD due to similarity in the structure and is the main psychoactive compound found in Claviceps Paspali and in (fresh) Heavenly Blue Morning Glory Seeds. LSH is unstable and breaks down into LSA quickly, so old seeds often only contains LSA and iso-LSA. When the seeds are fresh, they contain significantly higher amounts of LSH.

A sclerotium is a compact mass of hardened fungal mycelium containing food reserves. One role of sclerotia is to survive environmental extremes. In some higher fungi such as ergot, sclerotia become detached and remain dormant until favorable growth conditions return. Sclerotia initially were mistaken for individual organisms and described as separate species until Louis René Tulasne proved in 1853 that sclerotia are only a stage in the life cycle of some fungi. Further investigation showed that this stage appears in many fungi belonging to many diverse groups. Sclerotia are important in the understanding of the life cycle and reproduction of fungi, as a food source, as medicine, and in agricultural blight management.

Claviceps purpurea is an ergot fungus that grows on the ears of rye and related cereal and forage plants. Consumption of grains or seeds contaminated with the survival structure of this fungus, the ergot sclerotium, can cause ergotism in humans and other mammals. C. purpurea most commonly affects outcrossing species such as rye, as well as triticale, wheat and barley. It affects oats only rarely.

The Clavicipitaceae are a family of fungi within the order Hypocreales. A 2008 estimate placed 43 genera in the family, but a study in 2020 has increased this number to 50.

Ergocryptine is an ergopeptine and one of the ergoline alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine. β-Ergocryptine was first identified in 1967 by Albert Hofmann. Ergot from different sources have different ratios of the two isomers.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 μg/mL, ranging from 3.5 (NEC) to 45 (A549) μg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 synchronized cells at 1-5 μg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.

Stephacidin A and B are antitumor alkaloids isolated from the fungus Aspergillus ochraceus that belong to a class of naturally occurring 2,5-diketopiperazines. This unusual family of fungal metabolites are complex bridged 2,5-diketopiperazine alkaloids that possess a unique bicyclo[2.2.2]diazaoctane core ring system and are constituted mainly from tryptophan, proline, and substituted proline derivatives where the olefinic unit of the isoprene moiety has been formally oxidatively cyclized across the α-carbon atoms of a 2,5-diketopiperazine ring. The molecular architecture of stephacidin B, formally a dimer of avrainvillamide, reveals a complex dimeric prenylated N-hydroxyindole alkaloid that contains 15 rings and 9 stereogenic centers and is one of the most complex indole alkaloids isolated from fungi. Stephacidin B rapidly converts into the electrophilic monomer avrainvillamide in cell culture, and there is evidence that the monomer avrainvillamide interacts with intracellular thiol-containing proteins, most likely by covalent modification.

Dideoxyverticillin A, also known as (+)-11,11′-dideoxyverticillin A, is a complex epipolythiodioxopiperazine initially isolated from the marine fungus Penicillium sp. in 1999. It has also been found in the marine fungus Bionectriaceae, and belongs to a class of naturally occurring 2,5-diketopiperazines.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Penicillium vinaceum is an anamorph species of fungus in the genus Penicillium which produces penicillivinacine, vinaxanthone and citromycetin.

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.

The mycotoxin phomoxanthone A, or PXA for short, is a toxic natural product that affects the mitochondria. It is the most toxic and the best studied of the naturally occurring phomoxanthones. PXA has recently been shown to induce rapid, non-canonical mitochondrial fission by causing the mitochondrial matrix to fragment while the outer mitochondrial membrane can remain intact. This process was shown to be independent from the mitochondrial fission and fusion regulators DRP1 and OPA1.
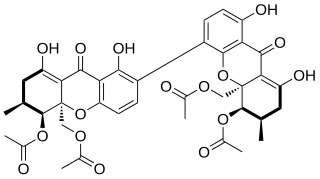
The mycotoxin phomoxanthone B, or PXB for short, is a toxic natural product. It is a less toxic isomer of phomoxanthone A and one of the two founding members of the class of phomoxanthone compounds. The phomoxanthones are named after the fungus Phomopsis, from which they were first isolated, and after their xanthonoid structure. Chemically, they are dimers of two tetrahydroxanthones that are covalently linked to each other. PXB itself is a homodimer of two identical diacetylated tetrahydroxanthones. The position of the link between the two tetrahydroxanthones is the only structural difference between PXB and its isomers PXA and dicerandrol C: In PXA, the two xanthonoid monomers are symmetrically linked at C-4,4’, while in PXB, they are asymmetrically linked at C-2,4’, and in dicerandrol C, they are symmetrically linked at C-2,2’.
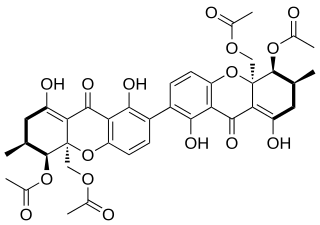
Dicerandrol C is a natural product. It is a less toxic isomer of phomoxanthone A (PXA) and phomoxanthone B (PXB), all three of which are members of the class of phomoxanthone compounds. The phomoxanthones are named after the fungus Phomopsis, from which they were first isolated, and after their xanthonoid structure. Chemically, they are dimers of two tetrahydroxanthones that are covalently linked to each other. Dicerandrol C itself is a homodimer of two identical diacetylated tetrahydroxanthones. The position of the link between the two tetrahydroxanthones is the only structural difference between dicerandrol C and its isomers PXA and PXB: In PXA, the two xanthonoid monomers are symmetrically linked at C-4,4’, while in PXB, they are asymmetrically linked at C-2,4’, and in dicerandrol C, they are symmetrically linked at C-2,2’.
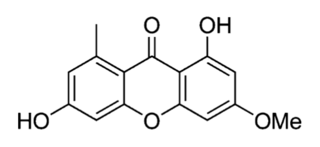
Griseoxanthone C is an organic compound in the structural class of chemicals known as xanthones. Its chemical formula is 1,6-dihydroxy-3-methoxy-8-methylxanthen-9-one, and its molecular formula is C15H12O5. It is found in a plant and some fungi, including a lichen.























