Related Research Articles

Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons and positively charged metal ions. It may be described as the sharing of free electrons among a structure of positively charged ions (cations). Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and lustre.
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like corrosion, and subtler effects associated with heterogeneous catalysis, where the catalyst and reactants are in different phases. The strong interaction between the adsorbate and the substrate surface creates new types of electronic bonds.
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption.
In physics, scattering is a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities in the medium through which they pass. In conventional use, this also includes deviation of reflected radiation from the angle predicted by the law of reflection. Reflections of radiation that undergo scattering are often called diffuse reflections and unscattered reflections are called specular (mirror-like) reflections. Originally, the term was confined to light scattering. As more "ray"-like phenomena were discovered, the idea of scattering was extended to them, so that William Herschel could refer to the scattering of "heat rays" in 1800. John Tyndall, a pioneer in light scattering research, noted the connection between light scattering and acoustic scattering in the 1870s. Near the end of the 19th century, the scattering of cathode rays and X-rays was observed and discussed. With the discovery of subatomic particles and the development of quantum theory in the 20th century, the sense of the term became broader as it was recognized that the same mathematical frameworks used in light scattering could be applied to many other phenomena.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.
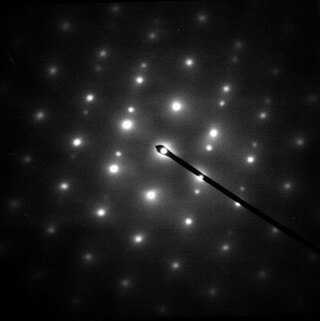
Electron diffraction is a generic term for phenomena associated with changes in the direction of electron beams due to elastic interactions with atoms. It occurs due to elastic scattering, when there is no change in the energy of the electrons. The negatively charged electrons are scattered due to Coulomb forces when they interact with both the positively charged atomic core and the negatively charged electrons around the atoms. The resulting map of the directions of the electrons far from the sample is called a diffraction pattern, see for instance Figure 1. Beyond patterns showing the directions of electrons, electron diffraction also plays a major role in the contrast of images in electron microscopes.

In the field of optics, transparency is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale, the photons can be said to follow Snell's law. Translucency allows light to pass through but does not necessarily follow Snell's law; the photons can be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color. The opposite property of translucency is opacity. Other categories of visual appearance, related to the perception of regular or diffuse reflection and transmission of light, have been organized under the concept of cesia in an order system with three variables, including transparency, translucency and opacity among the involved aspects.
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, integrated passive devices, light-emitting diodes, optical coatings, hard coatings on cutting tools, and for both energy generation and storage. It is also being applied to pharmaceuticals, via thin-film drug delivery. A stack of thin films is called a multilayer.
Absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter the liquid or solid bulk phase of a material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reagents or products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
Desorption is the physical process where adsorbed atoms or molecules are released from a surface into the surrounding vacuum or fluid. This occurs when a molecule gains enough energy to overcome the activation barrier and the binding energy that keep it attached to the surface.
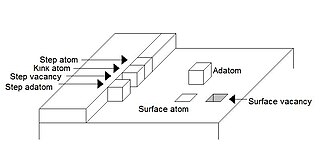
An adatom is an atom that lies on a crystal surface, and can be thought of as the opposite of a surface vacancy. This term is used in surface chemistry and epitaxy, when describing single atoms lying on surfaces and surface roughness. The word is a portmanteau of "adsorbed atom". A single atom, a cluster of atoms, or a molecule or cluster of molecules may all be referred to by the general term "adparticle". This is often a thermodynamically unfavorable state. However, cases such as graphene may provide counter-examples.
Helium atom scattering (HAS) is a surface analysis technique used in materials science. It provides information about the surface structure and lattice dynamics of a material by measuring the diffracted atoms from a monochromatic helium beam incident on the sample.

Low-energy ion scattering spectroscopy (LEIS), sometimes referred to simply as ion scattering spectroscopy (ISS), is a surface-sensitive analytical technique used to characterize the chemical and structural makeup of materials. LEIS involves directing a stream of charged particles known as ions at a surface and making observations of the positions, velocities, and energies of the ions that have interacted with the surface. Data that is thus collected can be used to deduce information about the material such as the relative positions of atoms in a surface lattice and the elemental identity of those atoms. LEIS is closely related to both medium-energy ion scattering (MEIS) and high-energy ion scattering, differing primarily in the energy range of the ion beam used to probe the surface. While much of the information collected using LEIS can be obtained using other surface science techniques, LEIS is unique in its sensitivity to both structure and composition of surfaces. Additionally, LEIS is one of a very few surface-sensitive techniques capable of directly observing hydrogen atoms, an aspect that may make it an increasingly more important technique as the hydrogen economy is being explored.
Selective chemistry of single-walled nanotubes is a field in Carbon nanotube chemistry devoted specifically to the study of functionalization of single-walled carbon nanotubes.

The Langmuir adsorption model explains adsorption by assuming an adsorbate behaves as an ideal gas at isothermal conditions. According to the model, adsorption and desorption are reversible processes. This model even explains the effect of pressure; i.e., at these conditions the adsorbate's partial pressure is related to its volume V adsorbed onto a solid adsorbent. The adsorbent, as indicated in the figure, is assumed to be an ideal solid surface composed of a series of distinct sites capable of binding the adsorbate. The adsorbate binding is treated as a chemical reaction between the adsorbate gaseous molecule and an empty sorption site S. This reaction yields an adsorbed species with an associated equilibrium constant :
Adsorption is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and as such they adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
The strength of metal oxide adhesion effectively determines the wetting of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites that depend on the optimization of wetting to create metal-ceramic interfaces. The strength of adhesion also determines the extent of dispersion on catalytically active metal. Metal oxide adhesion is important for applications such as complementary metal oxide semiconductor devices. These devices make possible the high packing densities of modern integrated circuits.
Helium-3 surface spin echo (HeSE) is an inelastic scattering technique in surface science that has been used to measure microscopic dynamics at well-defined surfaces in ultra-high vacuum. The information available from HeSE complements and extends that available from other inelastic scattering techniques such as neutron spin echo and traditional helium-4 atom scattering (HAS).
References
- ↑ Holden, Alan (1982). Crystals and crystal growing. MIT Press. ISBN 0-585-35966-0. OCLC 1289746352.
- Hulpke, E. (1992). Helium Atom Scattering from Surfaces. Springer. p. 168. ISBN 9783662027745.
- Torres, Zuleika Medina (2008). "Selective adsorbtion". Theoretical Studies of Gas-surface interactions. pp. 7–8.