Selenium oxide may refer to either of the following compounds:
- Selenium dioxide, SeO2
- Selenium trioxide, SeO3
- Diselenium pentoxide, Se2O5
Selenium oxide may refer to either of the following compounds:

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. It consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). The chemically uncharacterized synthetic element livermorium (Lv) is predicted to be a chalcogen as well. Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium—from Greek selḗnē —was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.
Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as (HO)2SeO2. It is a colorless compound. Although it has few uses, its derivative sodium selenate is used in the production of glass and animal feeds.
Selenium disulfide, also known as selenium sulfide, is a chemical compound and medication used to treat pityriasis versicolor, seborrhoeic dermatitis, and dandruff. It is applied to the affected area as a lotion or shampoo. Dandruff frequently returns if treatment is stopped.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.

The selenite anion is the oxyanion with the chemical formula SeO2−
3. It is the selenium analog of the sulfite ion, SO2−
3. Thus selenite is pyramidal and selenium is assigned oxidation state IV. A selenite (compound) is a compound that contains this ion. A common source of selenite is sodium selenite.

Selenous acid is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by (HO)2SeO. It is the principal oxoacid of selenium; the other being selenic acid.

Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound.
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
Selenium oxydichloride is the inorganic compound with the formula SeOCl2. It is a colorless liquid. With a high dielectric constant (55) and high specific conductance, it is an attractive solvent. Structurally, it is a close chemical relative of thionyl chloride SOCl2, being a pyramidal molecule.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.
Selenite may refer to:
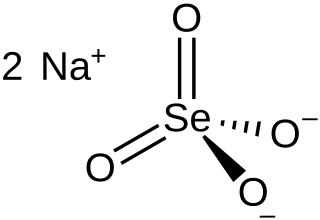
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Selenium tetrachloride is the inorganic compound composed with the formula SeCl4. This compound exists as yellow to white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds.
Selenium sulfide can refer to either of the following:

Selenium monochloride is an inorganic compound with the formula Se2Cl2. Although it is called selenium monochloride, a more descriptive name might be diselenium dichloride. It is a reddish-brown, oily liquid that hydrolyses slowly. It exists in chemical equilibrium with SeCl2, SeCl4, chlorine, and elemental selenium. Selenium monochloride is mainly used as a reagent for the synthesis of Se-containing compounds.

Selenoyl fluoride, selenoyl difluoride, selenium oxyfluoride, or selenium dioxydifluoride is a chemical compound with the formula SeO2F2.
Selenium iodide is a binary inorganic compound of selenium and iodine with the chemical formula Se
2I
2. The compound decomposes in water.