
Cell junctions or junctional complexes are a class of cellular structures consisting of multiprotein complexes that provide contact or adhesion between neighboring cells or between a cell and the extracellular matrix in animals. They also maintain the paracellular barrier of epithelia and control paracellular transport. Cell junctions are especially abundant in epithelial tissues. Combined with cell adhesion molecules and extracellular matrix, cell junctions help hold animal cells together.

Tight junctions, also known as occluding junctions or zonulae occludentes, are multiprotein junctional complexes whose canonical function is to prevent leakage of solutes and water and seals between the epithelial cells. They also play a critical role maintaining the structure and permeability of endothelial cells. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. The corresponding junctions that occur in invertebrates are septate junctions.

Claudins are a family of proteins which, along with occludin, are the most important components of the tight junctions. Tight junctions establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium. They have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm.

Occludin is a transmembrane protein that regulates the permeability of epithelial and endothelial barriers. It was first identified in epithelial cells as a 65 kDa integral plasma-membrane protein localized at the tight junctions. Together with Claudins, and zonula occludens-1 (ZO-1), occludin has been considered a staple of tight junctions, and although it was shown to regulate the formation, maintenance, and function of tight junctions, its precise mechanism of action remained elusive and most of its actions were initially attributed to conformational changes following selective phosphorylation, and its redox-sensitive dimerization. However, mounting evidence demonstrated that occludin is not only present in epithelial/endothelial cells, but is also expressed in large quantities in cells that do not have tight junctions but have very active metabolism: pericytes, neurons and astrocytes, oligodendrocytes, dendritic cells, monocytes/macrophages lymphocytes, and myocardium. Recent work, using molecular modeling, supported by biochemical and live-cell experiments in human cells demonstrated that occludin is a NADH oxidase that influences critical aspects of cell metabolism like glucose uptake, ATP production and gene expression. Furthermore, manipulation of occludin content in human cells is capable of influencing the expression of glucose transporters, and the activation of transcription factors like NFkB, and histone deacetylases like sirtuins, which proved capable of diminishing HIV replication rates in infected human macrophages under laboratory conditions.

Claudin-1 is a protein that in humans is encoded by the CLDN1 gene. It belongs to the group of claudins.

Claudin 4, also known as CLDN4, is a protein which in humans is encoded by the CLDN4 gene. It belongs to the group of claudins.
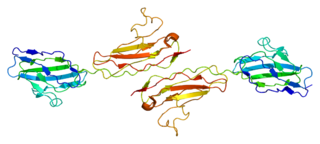
Junctional adhesion molecule A is a protein that in humans is encoded by the F11R gene. It has also been designated as CD321.

Claudin-5 is a protein that in humans is encoded by the CLDN5 gene. It belongs to the group of claudins.

Claudin 3, also known as CLDN3, is a protein which in humans is encoded by the CLDN3 gene. It is a member of the claudin protein family.

Claudin-7 is a protein that in humans is encoded by the CLDN7 gene. It belongs to the group of claudins.

Claudin-6 is a protein that in humans is encoded by the CLDN6 gene. It belongs to the group of claudins. The knockout mice of mouse homolog exhibit no phenotype, indicating that claudin-6 is dispensable for normal development and homeostasis.

Claudin-2 is a protein that in humans is encoded by the CLDN2 gene. It belongs to the group of claudins.

Claudin-11 is a protein that in humans is encoded by the CLDN11 gene. It belongs to the group of claudins and was the first member of the family to be knocked out in mice, thereby demonstrating the central role of claudins for intramembranous strands observed in freeze-fracture images.

Claudin-16 is a protein that in humans is encoded by the CLDN16 gene. It belongs to the group of claudins.

Claudin-14 is a protein that in humans is encoded by the CLDN14 gene. It belongs to a related family of proteins called claudins.

Claudin-9 is a protein that in humans is encoded by the CLDN9 gene. It belongs to the group of claudins.

Cingulin is a cytosolic protein encoded by the CGN gene in humans localized at tight junctions (TJs) of vertebrate epithelial and endothelial cells.

Claudin-10 is a protein that in humans is encoded by the CLDN10 gene. It belongs to the group of claudins.

Claudin-15 is a protein that in humans is encoded by the CLDN15 gene. It belongs to the group of claudins. Among its related pathways are Blood-Brain Barrier and Immune Cell Transmigration: VCAM-1/CD106 Signaling Pathways and Tight junction. GO annotations related to this gene include identical protein binding and structural molecule activity. An important paralog of this gene is CLDN10.
Tight junction proteins are molecules situated at the tight junctions of epithelial, endothelial and myelinated cells. This multiprotein junctional complex has a regulatory function in passage of ions, water and solutes through the paracellular pathway. It can also coordinate the motion of lipids and proteins between the apical and basolateral surfaces of the plasma membrane. Thereby tight junction conducts signaling molecules, that influence the differentiation, proliferation and polarity of cells. So tight junction plays a key role in maintenance of osmotic balance and trans-cellular transport of tissue specific molecules. Nowadays is known more than 40 different proteins, that are involved in these selective TJ channels.













