Related Research Articles

Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick.
In materials science, a metal matrix composite (MMC) is a composite material with fibers or particles dispersed in a metallic matrix, such as copper, aluminum, or steel. The secondary phase is typically a ceramic or another metal. They are typically classified according to the type of reinforcement: short discontinuous fibers (whiskers), continuous fibers, or particulates. There is some overlap between MMCs and cermets, with the latter typically consisting of less than 20% metal by volume. When at least three materials are present, it is called a hybrid composite. MMCs can have much higher strength-to-weight ratios, stiffness, and ductility than traditional materials, so they are often used in demanding applications. MMCs typically have lower thermal and electrical conductivity and poor resistance to radiation, limiting their use in the very harshest environments.

Silicon carbide (SiC), also known as carborundum, is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.

Ceramic engineering is the science and technology of creating objects from inorganic, non-metallic materials. This is done either by the action of heat, or at lower temperatures using precipitation reactions from high-purity chemical solutions. The term includes the purification of raw materials, the study and production of the chemical compounds concerned, their formation into components and the study of their structure, composition and properties.
Chemical vapour infiltration (CVI) is a ceramic engineering process whereby matrix material is infiltrated into fibrous preforms by the use of reactive gases at elevated temperature to form fiber-reinforced composites. The earliest use of CVI was the infiltration of fibrous alumina with chromium carbide. CVI can be applied to the production of carbon-carbon composites and ceramic-matrix composites. A similar technique is chemical vapour deposition (CVD), the main difference being that the deposition of CVD is on hot bulk surfaces, while CVI deposition is on porous substrates.

Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and hafnium diboride.

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.

In materials science ceramic matrix composites (CMCs) are a subgroup of composite materials and a subgroup of ceramics. They consist of ceramic fibers embedded in a ceramic matrix. The fibers and the matrix both can consist of any ceramic material, including carbon and carbon fibers.
AlSiC, pronounced "alsick", is a metal matrix composite consisting of aluminium matrix with silicon carbide particles. It has high thermal conductivity, and its thermal expansion can be adjusted to match other materials, e.g. silicon and gallium arsenide chips and various ceramics. It is chiefly used in microelectronics as substrate for power semiconductor devices and high density multi-chip modules, where it aids with removal of waste heat.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).
Trimethylsilane is the organosilicon compound with the formula (CH3)3SiH. It is a trialkylsilane. The Si-H bond is reactive. It is less commonly used as a reagent than the related triethylsilane, which is a liquid at room temperature.
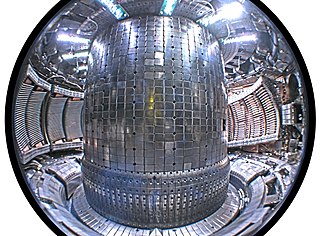
In nuclear fusion power research, the plasma-facing material (PFM) is any material used to construct the plasma-facing components (PFC), those components exposed to the plasma within which nuclear fusion occurs, and particularly the material used for the lining the first wall or divertor region of the reactor vessel.
Ultra-high-temperature ceramics (UHTCs) are a type of refractory ceramics that can withstand extremely high temperatures without degrading, often above 2,000 °C. They also often have high thermal conductivities and are highly resistant to thermal shock, meaning they can withstand sudden and extreme changes in temperature without cracking or breaking. Chemically, they are usually borides, carbides, nitrides, and oxides of early transition metals.
SiC–SiC matrix composite is a particular type of ceramic matrix composite (CMC) which have been accumulating interest mainly as high temperature materials for use in applications such as gas turbines, as an alternative to metallic alloys. CMCs are generally a system of materials that are made up of ceramic fibers or particles that lie in a ceramic matrix phase. In this case, a SiC/SiC composite is made by having a SiC matrix phase and a fiber phase incorporated together by different processing methods. Outstanding properties of SiC/SiC composites include high thermal, mechanical, and chemical stability while also providing high strength to weight ratio.
Boron fiber or boron filament is an amorphous product which represents the major industrial use of elemental boron. Boron fiber manifests a combination of high strength and high elastic modulus.
Laser chemical vapor deposition (LCVD) is a chemical process used to produce high purity, high performance films, fibers, and mechanical hardware (MEMS). It is a form of chemical vapor deposition in which a laser beam is used to locally heat the semiconductor substrate, causing the vapor deposition chemical reaction to proceed faster at that site. The process is used in the semiconductor industry for spot coating, the MEMS industry for 3-D printing of hardware such as springs and heating elements,2,6,7,9 and the composites industry for boron and ceramic fibers. As with conventional CVD, one or more gas phase precursors are thermally decomposed, and the resulting chemical species 1) deposit on a surface, or 2) react, form the desired compound, and then deposit on a surface, or a combination of (1) and (2).
Ultra-high temperature ceramic matrix composites (UHTCMC) are a class of refractory ceramic matrix composites (CMCs) with melting points significantly higher than that of typical CMCs. Among other applications, they are the subject of extensive research in the aerospace engineering field for their ability to withstand extreme heat for extended periods of time, a crucial property in applications such as thermal protection systems (TPS) and rocket nozzles. Carbon fiber-reinforced carbon (C/C) maintains its structural integrity up to 2000 °C; however, C/C is mainly used as an ablative material, designed to purposefully erode under extreme temperatures in order to dissipate energy. Carbon fiber reinforced silicon carbide matrix composites (C/SiC) and Silicon carbide fiber reinforced silicon carbide matrix composites (SiC/SiC) are considered reusable materials because silicon carbide is a hard material with a low erosion and it forms a silica glass layer during oxidation which prevents further oxidation of inner material. However, above a certain temperature starts the active oxidation of silicon carbide matrix to gaseous silicon monoxide, consequently loss of protection from further oxidation, which leads the material to an uncontrolled and fast erosion. For this reason C/SiC and SiC/SiC are used in the range of temperature between 1200° - 1400 °C.
References
- 1 2 3 4 5 Composite Materials Handbook (CMH-17) Volume 5, Ceramic Matrix Composites; published by SAE International, 2017 (from chapter 3.2) https://www.library.ucdavis.edu/wp-content/uploads/2016/12/HDBK17-5.pdf
- 1 2 3 4 https://www.library.ucdavis.edu/wp-content/uploads/2017/03/HDBK17-3F.pdf section 2.4.1.6
- ↑ "Ceramic matrix composites take flight in LEAP jet engine | ORNL". Ornl.gov. 2017-01-03. Retrieved 2018-03-30.
- ↑ Website of CFM, the joint venture manufacturer (between GE and Saffran Aircraft Engines) of the LEAP engine, https://www.cfmaeroengines.com/engines/leap/
- ↑ Heiss, Christian; Travitzky, Nahum; Greil, Peter (2012-07-03). "Manufacturing of Silicon Carbide Knit Fabrics". Advanced Engineering Materials. 14 (3): 162–165. doi:10.1002/adem.201100192. S2CID 135924275.
- ↑ "Silicon Carbide SiC Material Properties". Accuratus.com. Retrieved 2018-03-30.
- ↑ US 4,052,430,Yajima, Seishi; Hayashi, Josaburo& Omori, Mamoru,"Method for producing organosilicon high molecular weight compounds having silicon and carbon as main skeleton components and said organosilicon high molecular weight compounds",issued 1977-10-04
- ↑ "Specialty Materials, Inc. - History". Specmaterials.com. Retrieved 2018-03-30.
- ↑ Maxwell, James L.; Chavez, Craig A.; Springer, Robert W.; Maskaly, Karlene R.; Goodin, Dan (2007). "Preparation of superhard BxCy fibers by microvortex-flow hyperbaric laser chemical vapor deposition". Diamond and Related Materials. 16 (8): 1557–1564. doi:10.1016/j.diamond.2006.12.059.
- ↑ Wallenberger, Frederick T.; Nordine, Paul C.; Boman, Mats (1994). "Inorganic fibers and microstructures directly from the vapor phase". Composites Science and Technology. 51 (2): 193–212. doi:10.1016/0266-3538(94)90190-2.
- ↑ Maxwell, James L.; Boman, Mats; Springer, Robert W.; Narayan, Jaikumar; Gnanavelu, Saiprasanna (2006). "Hyperbaric Laser Chemical Vapor Deposition of Carbon Fibers from the 1-Alkenes, 1-Alkynes, and Benzene". Journal of the American Chemical Society. 128 (13): 4405–4413. doi:10.1021/ja057666j. PMID 16569018.
- ↑ "Silicon Carbide (SiC) Fiber-Reinforced SiC Matrix Composites | T2 Portal". technology.nasa.gov. Retrieved 2022-03-04.
- ↑ US 20120088088A1
- ↑ Ross, Lisa (Aug 8, 2024). "What Are the Uses of Silicon Carbide?". Advanced Ceramic Materials. Retrieved Sep 23, 2024.
- ↑ Agarwal, A.K. (1996). "SiC electronics". International Electron Devices Meeting. Technical Digest. pp. 225–230. doi:10.1109/IEDM.1996.553573. ISBN 0-7803-3393-4.