Related Research Articles

Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics. The word crystallography is derived from the Ancient Greek word κρύσταλλος, with its meaning extending to all solids with some degree of transparency, and γράφειν. In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography.

X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information.

A synchrotron light source is a source of electromagnetic radiation (EM) usually produced by a storage ring, for scientific and technical purposes. First observed in synchrotrons, synchrotron light is now produced by storage rings and other specialized particle accelerators, typically accelerating electrons. Once the high-energy electron beam has been generated, it is directed into auxiliary components such as bending magnets and insertion devices in storage rings and free electron lasers. These supply the strong magnetic fields perpendicular to the beam that are needed to stimulate the high energy electrons to emit photons.
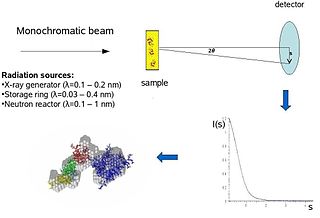
Biological small-angle scattering is a small-angle scattering method for structure analysis of biological materials. Small-angle scattering is used to study the structure of a variety of objects such as solutions of biological macromolecules, nanocomposites, alloys, and synthetic polymers. Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) are the two complementary techniques known jointly as small-angle scattering (SAS). SAS is an analogous method to X-ray and neutron diffraction, wide angle X-ray scattering, as well as to static light scattering. In contrast to other X-ray and neutron scattering methods, SAS yields information on the sizes and shapes of both crystalline and non-crystalline particles. When used to study biological materials, which are very often in aqueous solution, the scattering pattern is orientation averaged.
In physics, the phase problem is the problem of loss of information concerning the phase that can occur when making a physical measurement. The name comes from the field of X-ray crystallography, where the phase problem has to be solved for the determination of a structure from diffraction data. The phase problem is also met in the fields of imaging and signal processing. Various approaches of phase retrieval have been developed over the years.

Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while Se-methylselenocysteine, or its γ-glutamyl derivative, is the major form of selenium found in Astragalus, Allium, and Brassica species. In vivo, selenomethionine is randomly incorporated instead of methionine. Selenomethionine is readily oxidized.
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope (TEM). It can involve the use of high-resolution transmission electron microscopy images, electron diffraction patterns including convergent-beam electron diffraction or combinations of these. It has been successful in determining some bulk structures, and also surface structures. Two related methods are low-energy electron diffraction which has solved the structure of many surfaces, and reflection high-energy electron diffraction which is used to monitor surfaces often during growth.
A diffractometer is a measuring instrument for analyzing the structure of a material from the scattering pattern produced when a beam of radiation or particles interacts with it.
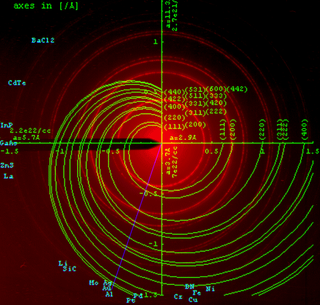
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer.

The Stanford Synchrotron Radiation Lightsource, a division of SLAC National Accelerator Laboratory, is operated by Stanford University for the Department of Energy. SSRL is a National User Facility which provides synchrotron radiation, a name given to electromagnetic radiation in the x-ray, ultraviolet, visible and infrared realms produced by electrons circulating in a storage ring at nearly the speed of light. The extremely bright light that is produced can be used to investigate various forms of matter ranging from objects of atomic and molecular size to man-made materials with unusual properties. The obtained information and knowledge is of great value to society, with impact in areas such as the environment, future technologies, health, biology, basic research, and education.
The National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory (BNL) in Upton, New York was a national user research facility funded by the U.S. Department of Energy (DOE). Built from 1978 through 1984, and officially shut down on September 30, 2014, the NSLS was considered a second-generation synchrotron.

ALBA is a third-generation synchrotron light source facility located in the Barcelona Synchrotron Park in Cerdanyola del Vallès near Barcelona, in Catalonia (Spain). It was constructed and is operated by CELLS, and co-financed by the Spanish central administration and regional Catalan Government.

Fiber diffraction is a subarea of scattering, an area in which molecular structure is determined from scattering data. In fiber diffraction the scattering pattern does not change, as the sample is rotated about a unique axis. Such uniaxial symmetry is frequent with filaments or fibers consisting of biological or man-made macromolecules. In crystallography fiber symmetry is an aggravation regarding the determination of crystal structure, because reflexions are smeared and may overlap in the fiber diffraction pattern. Materials science considers fiber symmetry a simplification, because almost the complete obtainable structure information is in a single two-dimensional (2D) diffraction pattern exposed on photographic film or on a 2D detector. 2 instead of 3 co-ordinate directions suffice to describe fiber diffraction.
Multi-wavelength anomalous diffraction is a technique used in X-ray crystallography that facilitates the determination of the three-dimensional structure of biological macromolecules via solution of the phase problem.
Diffraction topography is a imaging technique based on Bragg diffraction. Diffraction topographic images ("topographies") record the intensity profile of a beam of X-rays diffracted by a crystal. A topography thus represents a two-dimensional spatial intensity mapping of reflected X-rays, i.e. the spatial fine structure of a Laue reflection. This intensity mapping reflects the distribution of scattering power inside the crystal; topographs therefore reveal the irregularities in a non-ideal crystal lattice. X-ray diffraction topography is one variant of X-ray imaging, making use of diffraction contrast rather than absorption contrast which is usually used in radiography and computed tomography (CT). Topography is exploited to a lesser extends with neutrons, and has similarities to dark field imaging in the electron microscope community.

Johannes Martin Bijvoet was a Dutch chemist and crystallographer at the van 't Hoff Laboratory at Utrecht University. He is famous for devising a method of establishing the absolute configuration of molecules. In 1946, he became member of the Royal Netherlands Academy of Arts and Sciences.
Anomalous X-ray scattering is a non-destructive determination technique within X-ray diffraction that makes use of the anomalous dispersion that occurs when a wavelength is selected that is in the vicinity of an absorption edge of one of the constituent elements of the sample. It is used in materials research to study nanometer sized differences in structure.
Isomorphous replacement (IR) is historically the most common approach to solving the phase problem in X-ray crystallography studies of proteins. For protein crystals this method is conducted by soaking the crystal of a sample to be analyzed with a heavy atom solution or co-crystallization with the heavy atom. The addition of the heavy atom (or ion) to the structure should not affect the crystal formation or unit cell dimensions in comparison to its native form, hence, they should be isomorphic.
Lieselotte Templeton was a German-born American crystallographer. She received the Patterson Award of the American Crystallographic Association together with her husband David H. Templeton in 1987.
This is a timeline of crystallography.
References
- 1 2 "Dictionary of common terms used in PHENIX". phenix-online.org.
MAD: [...] The differences in anomalous scattering around the edge allow calculation of phase angles without the phase ambiguity present in SAD experiments, although density modification will usually still be necessary to obtain an easily interpretable map. [...] Although very powerful, MAD phasing has declined somewhat in popularity relative to SAD because of the more limited choice of heavy atoms, the difficulty of avoiding radiation damage, and the requirement for a synchrotron beamline. [...] SAD: [...] SAD is often performed with selenomethionine-incorporated protein, but any anomalously scattering atom (including sulfur, if the data are of very high quality) may be used.
Further reading
- W. A. Hendrickson (1985). "Analysis of Protein Structure from Diffraction Measurement at Multiple Wavelengths". Trans. ACA Vol 21.
- J Karle (1980). "Some Developments in Anomalous Dispersion for the Structural Investigation of Macromolecular Systems in Biology". International Journal of Quantum Chemistry: Quantum Biology Symposium 7, 357–367.
- J. Karle (1989). "Linear Algebraic Analyses of Structures with One Predominant Type of Anomalous Scatterer". Acta Crystallogr. A45, 303–307.
- A. Pahler, JL Smith & WA Hendrickson (1990). "A Probability Representation for Phase Information from Multiwavelength Anomalous Dispersion". Acta Crystallogr. A46, 537–540.
- T. C. Terwilliger (1994). "MAD Phasing: Bayesian Estimates of FA" Acta Crystallogr. D50, 11–16.
- T. C. Terwilliger (1994). "MAD Phasing: Treatment of Dispersive Differences as Isomorphous Replacement Information" Acta Crystallogr. D50, 17–23.
- R. Fourme, W. Shepard, R. Kahn, G l'Hermite & IL de La Sierra (1995). "The Multiwavelength Anomalous Solvent Contrast (MASC) Method in Macrocolecular Crystallography". J. Synchrotron Rad. 2, 36–48.
- E. de la Fortelle and G. Bricogne (1997) "Maximum-Likelihood Heavy-Atom Parameter Refinement for Multiple Isomorphous Replacement and Multiwavelength Anomalous Diffraction Methods". Methods in Enzymology 276, 472–494.
- W. A. Hendrickson and CM Ogata (1997) "Phase Determination from Multiwavelength Anomalous Diffraction Measurements". Methods in Enzymology 276, 494–523.
- J. Bella & M. G. Rossmann (1998). "A General Phasing Algorithm for Multiple MAD and MIR Data" Acta Crystallogr. D54, 159–174.
- J. M. Guss, E. A. Merritt, R. P. Phizackerley, B. Hedman, M. Murata, K. O. Hodgson, and H. C. Freeman (1989). "Phase determination by multiple-wavelength X-ray diffraction: crystal structure of a basic blue copper protein from cucumbers". Science 241, 806–811.
- B. Vijayakumar and D. Velmurugan (2013). "Use of europium ions for SAD phasing of lysozyme at the Cu Kα wavelength" Acta Crystallogr. F69, 20–24.
- J. P. Rose & B-C Wang (2016) "SAD phasing: History, current impact and future opportunities" Archives Biochem Biophys 602, 80-94.