Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.
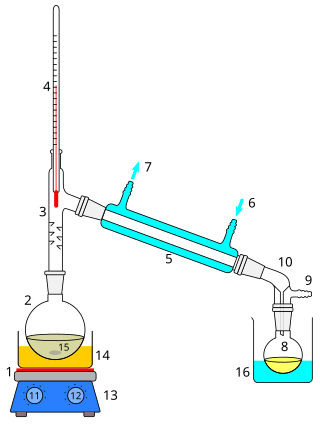
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products ; this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes the following applications:

A thermometer is a device that measures temperature or a temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.
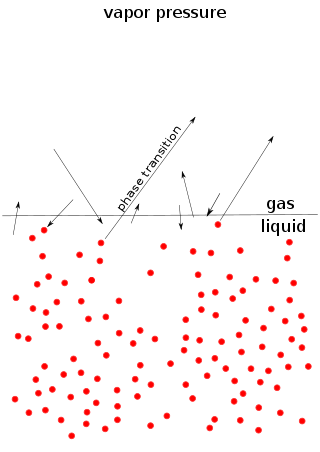
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.

Boiling is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned. Additionally, the reference sample must be stable, of high purity, and must not experience much change across the temperature scan. Typically, reference standards have been metals such as indium, tin, bismuth, and lead, but other standards such as polyethylene and fatty acids have been proposed to study polymers and organic compounds, respectively.

A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom.

Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The nanoparticles in the sintered material diffuse across the boundaries of the particles, fusing the particles together and creating a solid piece.
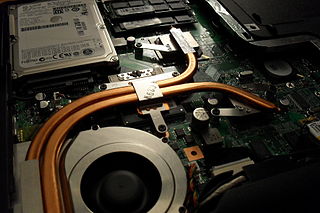
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

A boiling liquid expanding vapor explosion is an explosion caused by the rupture of a vessel containing a pressurized liquid that has reached a temperature above its boiling point. Because the boiling point of a liquid rises with pressure, the contents of the pressurized vessel can remain a liquid as long as the vessel is intact. If the vessel's integrity is compromised, the loss of pressure drops the boiling point, which can cause the liquid to convert to a gas expanding rapidly. If the gas is combustible, as in the case with hydrocarbons and alcohols, further damage can be caused by the ensuing fire.

A rotary evaporator (rotavap) is a device used in chemical laboratories for the efficient and gentle removal of solvents from samples by evaporation. When referenced in the chemistry research literature, description of the use of this technique and equipment may include the phrase "rotary evaporator", though use is often rather signaled by other language.

A hot plate is a portable self-contained tabletop small appliance cooktop that features one or more electric heating elements or gas burners. A hot plate can be used as a stand-alone appliance, but is often used as a substitute for one of the burners from an oven range or a kitchen stove. Hot plates are often used for food preparation, generally in locations where a full kitchen stove would not be convenient or practical. They can also be used as a heat source in laboratories. A hot plate can have a flat surface or round surface. Hot plates can be used for traveling or in areas without electricity.
Coal analysis techniques are specific analytical methods designed to measure the particular physical and chemical properties of coals. These methods are used primarily to determine the suitability of coal for coking, power generation or for iron ore smelting in the manufacture of steel.

The Victor Meyer apparatus is the standard laboratory method for determining the molecular weight of a volatile liquid. It was developed by Viktor Meyer, who spelled his name Victor in publications at the time of its development. In this method, a known mass of a volatile solid or liquid under examination is converted into its vapour form by heating in a Victor Meyer's tube. The vapour displaces its own volume of air. The volume of air displaced at experimental temperature and pressure is calculated. Then volume of air displaced at standard temperature and pressure is calculated. Using this, mass of air displaced at 2.24 × 10−2 m3 of vapour at STP is calculated. This value represents the molecular mass of the substance. The apparatus consists of an inner Victor Meyer's tube, lower end of which is in form of a bulb. The upper end of tube has a side tube that leads to a trough filled with water. The Victor Meyer's tube is surrounded by an outer jacket. In the outer jacket, a liquid is placed, which boils at a temperature at least 30 K higher than the substance under examination. A small quantity of glass-wool or asbestos pad covers the lower end of the Victor Meyer's tube to prevent breakage, when a glass bottle containing the substance under examination is dropped to it

Thermospray is a soft ionization source by which a solvent flow of liquid sample passes through a very thin heated column to become a spray of fine liquid droplets. As a form of atmospheric pressure ionization in mass spectrometry these droplets are then ionized via a low-current discharge electrode to create a solvent ion plasma. A repeller then directs these charged particles through the skimmer and acceleration region to introduce the aerosolized sample to a mass spectrometer. It is particularly useful in liquid chromatography-mass spectrometry (LC-MS).

The Thiele tube, named after the German chemist Johannes Thiele, is a laboratory glassware designed to contain and heat an oil bath. Such a setup is commonly used in the determination of the melting point or boiling point of a substance. The apparatus resembles a glass test tube with an attached handle.

A melting-point apparatus is a scientific instrument used to determine the melting point of a substance. Some types of melting-point apparatuses include the Thiele tube, Fisher-Johns apparatus, Gallenkamp (Electronic) melting-point apparatus and automatic melting-point apparatus.
Bumping is a phenomenon in chemistry where homogeneous liquids boiled in a test tube or other container will superheat and, upon nucleation, rapid boiling will expel the liquid from the container. In extreme cases, the container may be broken.
References
- ↑ Siwoloboff, A. (1886). "Ueber die Siedepunktbestimmung kleiner Mengen Flüssigkeiten" [About determining the boiling point of small amounts of liquids](PDF). Berichte der Deutschen Chemischen Gesellschaft. 19 (1): 795–796. doi:10.1002/cber.188601901181.
- ↑ "OECD Guideline for the Testing of Chemicals" (PDF). Retrieved 2023-10-22.
- ↑ Hunt, Ian R. (2021-09-27). "Micro-boiling point measurement" (PDF). University of Calgary . Retrieved 2023-10-22.
- ↑ Nichols, Lisa (2022-05-05). "Step-by-Step Procedures for Boiling Point Determination". LibreTexts. Retrieved 2023-10-22.