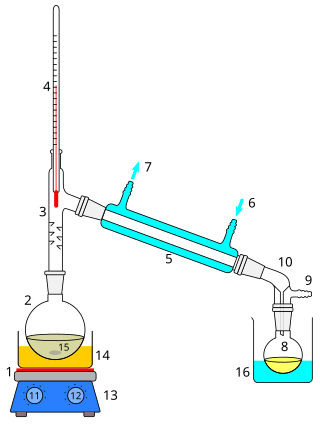
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

A thermometer is a device that measures temperature or temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.

Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment.

A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom.

The mercury-in-glass or mercury thermometer is a thermometer that uses the thermal expansion and contraction of liquid mercury to indicate the temperature.

Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
The Newton scale is a temperature scale devised by Isaac Newton in 1701. He called his device a "thermometer", but he did not use the term "temperature", speaking of "degrees of heat" instead. Newton's publication represents the first attempt to introduce an objective way of measuring temperature . Newton likely developed his scale for practical use rather than for a theoretical interest in thermodynamics; he had been appointed Warden of the Mint in 1695, and Master of the Mint in 1699, and his interest in the melting points of metals are likely inspired by his duties in connection with the Royal Mint.
The dropping point of a lubricating grease is an indication of the heat resistance of the grease and is the temperature at which it passes from a semi-solid to a liquid state under specific test conditions. It is dependent on the type of thickener used and the cohesiveness of the oil and thickener of a grease. The dropping point indicates the upper temperature limit at which a grease retains its structure though is not necessarily the maximum temperature at which a grease can be used.

A deep fryer is a kitchen appliance used for deep frying. Deep frying is a method of cooking by submerging food into oil at high heat, typically between temperatures of 350 to 375 °F.

Rainer Ludwig Claisen was a German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn (1869), where he became a member of K.St.V. Arminia. He served in the army as a nurse in 1870–1871 and continued his studies at Göttingen University. He returned to the University of Bonn in 1872 and started his academic career at the same university in 1874. He died in 1930 in Godesberg am Rhein.

A melting-point apparatus is a scientific instrument used to determine the melting point of a substance. Some types of melting-point apparatuses include the Thiele tube, Fisher-Johns apparatus, Gallenkamp (Electronic) melting-point apparatus and automatic melting-point apparatus.
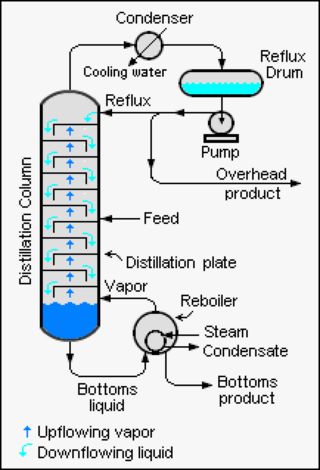
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.

Soldering is a process of joining two metal surfaces together using a filler metal called solder. The soldering process involves heating the surfaces to be joined and melting the solder, which is then allowed to cool and solidify, creating a strong and durable joint.

A water bath is laboratory equipment made from a container filled with heated water. It is used to incubate samples in water at a constant temperature over a long period of time. Most water baths have a digital or an analogue interface to allow users to set a desired temperature, but some water baths have their temperature controlled by a current passing through a reader.

The Siwoloboff method is used to determine the boiling point of small samples of liquid chemicals. A sample in an ignition tube is attached to a thermometer with a rubber band, and immersed in a Thiele tube, water bath, or other suitable medium for heating. A sealed capillary, open end pointing down, is placed in the ignition tube.

















