
Laboratory glassware is a variety of equipment used in scientific work, traditionally made of glass. Glass may be blown, bent, cut, molded, or formed into many sizes and shapes. It is commonly used in chemistry, biology, and analytical laboratories. Many laboratories have training programs to demonstrate how glassware is used and to alert first–time users to the safety hazards involved with using glassware.
A burette is a graduated glass tube with a tap at one end, for delivering known volumes of a liquid, especially in titrations. It is a long, graduated glass tube, with a stopcock at its lower end and a tapered capillary tube at the stopcock's outlet. The flow of liquid from the tube to the burette tip is controlled by the stopcock valve.

A pipette is a type of laboratory tool commonly used in chemistry and biology to transport a measured volume of liquid, often as a media dispenser. Pipettes come in several designs for various purposes with differing levels of accuracy and precision, from single piece glass pipettes to more complex adjustable or electronic pipettes. Many pipette types work by creating a partial vacuum above the liquid-holding chamber and selectively releasing this vacuum to draw up and dispense liquid. Measurement accuracy varies greatly depending on the instrument.

A syringe is a simple reciprocating pump consisting of a plunger that fits tightly within a cylindrical tube called a barrel. The plunger can be linearly pulled and pushed along the inside of the tube, allowing the syringe to take in and expel liquid or gas through a discharge orifice at the front (open) end of the tube. The open end of the syringe may be fitted with a hypodermic needle, a nozzle or tubing to direct the flow into and out of the barrel. Syringes are frequently used in clinical medicine to administer injections, infuse intravenous therapy into the bloodstream, apply compounds such as glue or lubricant, and draw/measure liquids. There are also prefilled syringes.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.

A eudiometer is a laboratory device that measures the change in volume of a gas mixture following a physical or chemical change.

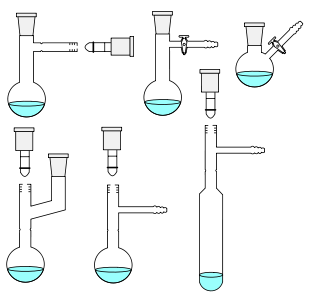
Laboratory flasks are vessels or containers that fall into the category of laboratory equipment known as glassware. In laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes, but a common distinguishing aspect in their shapes is a wider vessel "body" and one narrower tubular sections at the top called necks which have an opening at the top. Laboratory flask sizes are specified by the volume they can hold, typically in metric units such as milliliters or liters. Laboratory flasks have traditionally been made of glass, but can also be made of plastic.
The Marcusson apparatus, Dean-Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water from a reactor. It is used in combination with a reflux condenser and a distillation flask for the separation of water from liquids. This may be a continuous removal of the water that is produced during a chemical reaction performed at reflux temperature, such as in esterification reactions. The original setup by Julius Marcusson was refined by the American chemists Ernest Woodward Dean (1888–1959) and David Dewey Stark (1893–1979) in 1920 for determination of the water content in petroleum.

A dropping funnel or addition funnel is a type of laboratory glassware used to transfer liquids. They are fitted with a stopcock which allows the flow to be controlled. Dropping funnels are useful for adding reagents slowly, i.e. drop-wise. This is desirable when the quick addition of the reagent results in side reactions, or if the reaction is too vigorous.

Round-bottom flasks are types of flasks having spherical bottoms used as laboratory glassware, mostly for chemical or biochemical work. They are typically made of glass for chemical inertness; and in modern days, they are usually made of heat-resistant borosilicate glass. There is at least one tubular section known as the neck with an opening at the tip. Two- or three-necked flasks are common as well. Round bottom flasks come in many sizes, from 5 mL to 20 L, with the sizes usually inscribed on the glass. In pilot plants even larger flasks are encountered.

The Schlenk line is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is connected to a vacuum pump. The inert-gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the vacuum pump by a liquid-nitrogen or dry-ice/acetone cold trap. Special stopcocks or Teflon taps allow vacuum or inert gas to be selected without the need for placing the sample on a separate line.
A Schlenk flask, or Schlenk tube, is a reaction vessel typically used in air-sensitive chemistry, invented by Wilhelm Schlenk. It has a side arm fitted with a PTFE or ground glass stopcock, which allows the vessel to be evacuated or filled with gases. These flasks are often connected to Schlenk lines, which allow both operations to be done easily.
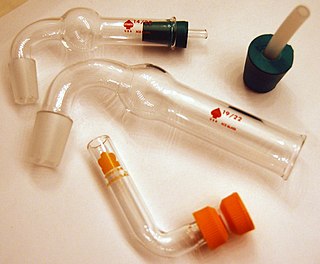
A drying tube or guard tube is a tube-like piece of apparatus used to house a disposable solid desiccant, wherein at one end the tube-like structure terminates in a ground glass joint for use in connecting the drying tube to a reaction vessel, for the purpose of keeping the vessel free of moisture.

Ground glass joints are used in laboratories to quickly and easily fit leak-tight apparatus together from interchangeable commonly available parts. For example, a round bottom flask, Liebig condenser, and oil bubbler with ground glass joints may be rapidly fitted together to reflux a reaction mixture. This is a large improvement compared with older methods of custom-made glassware, which was time-consuming and expensive, or the use of less chemical resistant and heat resistant corks or rubber bungs and glass tubes as joints, which took time to prepare as well.

An eye dropper, also called Pasteur pipette or simply dropper, is a device used to transfer small quantities of liquids. They are used in the laboratory and also to dispense small amounts of liquid medicines. A very common use was to dispense eye drops into the eye. The commonly recognized form is a glass tube tapered to a narrow point and fitted with a rubber bulb at the top, although many styles of both plastic and glass droppers exist. The combination of the pipette and rubber bulb has also been referred to as a teat pipette. The Pasteur pipette name is from the French scientist Louis Pasteur, who used a variant of them extensively during his research. In the past, there was no equipment to transfer a chemical solution without exposing it to the external environment. The hygiene and purity of chemical compounds is necessary for the expected result of each experiment. The eye dropper, both glass and plastic types, can be sterilized and plugged with a rubber bulb at the open end of the pipette preventing any contamination from the atmosphere. Generally, they are considered cheap enough to be disposable, however, so long as the glass point is not chipped, the eye dropper may be washed and reused indefinitely.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.

Cannula transfer or cannulation is a set of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. While the syringes are not the same as cannulae, the techniques remain relevant.

A media dispenser or a culture media dispenser is a device for repeatedly delivering small fixed volumes of liquid such as a laboratory growth medium like molten agar or caustic or volatile solvents like toluene into a series of receptacles. It is often important that such dispensers operate without biological or chemical contamination, and so must be internally sealed from the environment and designed for easy cleaning and sterilization before use. At a minimum, a media dispenser consists of some kind of pump connected to a length of discharge tubing or a spout. Dispensers used in laboratories are also frequently connected to microcontrollers to regulate the speed and volume of the medium as it leaves the pump.















