
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products. Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.

Dry ice is the solid form of carbon dioxide. It is commonly used as it does not have a liquid state and sublimates directly from the solid state to the gas state. It is used primarily as a cooling agent, but is also used in fog machines at theatres for dramatic effects. Its advantages include lower temperature than that of water ice and not leaving any residue. It is useful for preserving frozen foods where mechanical cooling is unavailable.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

A dehumidifier is an air conditioning device which reduces and maintains the level of humidity in the air. This is done usually for health or comfort reasons, or to eliminate musty odor and to prevent the growth of mildew by extracting water from the air. It can be used for household, commercial, or industrial applications. Large dehumidifiers are used in commercial buildings such as indoor ice rinks and swimming pools, as well as manufacturing plants or storage warehouses. Typical air conditioning systems combine dehumidification with cooling, by setting cooling coils below the dewpoint and collecting the condensate.

Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface.
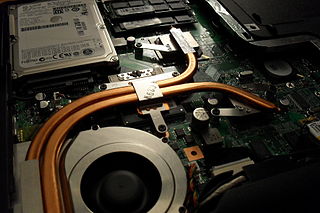
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.

Vacuum distillation is distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation.

A rotary evaporator (rotovap) is a device used in chemical laboratories for the efficient and gentle removal of solvents from samples by evaporation. When referenced in the chemistry research literature, description of the use of this technique and equipment may include the phrase "rotary evaporator", though use is often rather signaled by other language.

Freeze drying, also known as lyophilization or cryodesiccation, is a low temperature dehydration process that involves freezing the product, lowering pressure, then removing the ice by sublimation. This is in contrast to dehydration by most conventional methods that evaporate water using heat.

In vacuum applications, a cold trap is a device that condenses all vapors except the permanent gases into a liquid or solid. The most common objective is to prevent vapors being evacuated from an experiment from entering a vacuum pump where they would condense and contaminate it. Particularly large cold traps are necessary when removing large amounts of liquid as in freeze drying.

Steam distillation is a separation process that consists in distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container.
Auto-defrost, automatic defrost or self-defrosting is a technique which regularly defrosts the evaporator in a refrigerator or freezer. Appliances using this technique are often called frost free, frostless, or no-frost.
Drying is a mass transfer process consisting of the removal of water or another solvent by evaporation from a solid, semi-solid or liquid. This process is often used as a final production step before selling or packaging products. To be considered "dried", the final product must be solid, in the form of a continuous sheet, long pieces, particles or powder. A source of heat and an agent to remove the vapor produced by the process are often involved. In bioproducts like food, grains, and pharmaceuticals like vaccines, the solvent to be removed is almost invariably water. Desiccation may be synonymous with drying or considered an extreme form of drying.
The Dean–Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water from a reactor. It is used in combination with a reflux condenser and a batch reactor for continuous removal of the water that is produced during a chemical reaction performed at reflux temperature. It was invented by the American chemists Ernest Woodward Dean (1888–1959) and David Dewey Stark (1893–1979) in 1920 for determination of the water content in petroleum.

Round-bottom flasks are types of flasks having spherical bottoms used as laboratory glassware, mostly for chemical or biochemical work. They are typically made of glass for chemical inertness; and in modern days, they are usually made of heat-resistant borosilicate glass. There is at least one tubular section known as the neck with an opening at the tip. Two- or three-necked flasks are common as well. Round bottom flasks come in many sizes, from 5 mL to 20 L, with the sizes usually inscribed on the glass. In pilot plants even larger flasks are encountered.

Sublimation apparatus is equipment, commonly laboratory glassware, for purification of compounds by selective sublimation. In principle, the operation resembles purification by distillation, except that the products do not pass through a liquid phase.

A cooling bath or ice bath, in laboratory chemistry practice, is a liquid mixture which is used to maintain low temperatures, typically between 13 °C and −196 °C. These low temperatures are used to collect liquids after distillation, to remove solvents using a rotary evaporator, or to perform a chemical reaction below room temperature.

In chemistry, a condenser is laboratory apparatus used to condense vapors — that is, turn them into liquids — by cooling them down.

Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.


















