
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

An Erlenmeyer flask, also known as a conical flask or a titration flask, is a type of laboratory flask with a flat bottom, a conical body, and a cylindrical neck. It is named after the German chemist Emil Erlenmeyer (1825–1909), who invented it in 1860.

Laboratory glassware is a variety of equipment used in scientific work, traditionally made of glass. Glass may be blown, bent, cut, molded, or formed into many sizes and shapes. It is commonly used in chemistry, biology, and analytical laboratories. Many laboratories have training programs to demonstrate how glassware is used and to alert first–time users to the safety hazards involved with using glassware.

A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom.

A vacuum flask is an insulating storage vessel that slows the speed at which its contents change in temperature. It greatly lengthens the time over which its contents remain hotter or cooler than the flask's surroundings by trying to be as adiabatic as possible. Invented by Sir James Dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. The gap between the two flasks is partially evacuated of air, creating a near-vacuum which significantly reduces heat transfer by conduction or convection. When used to hold cold liquids, this also virtually eliminates condensation on the outside of the flask.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

A jar is a rigid, cylindrical or slightly conical container, typically made of glass, ceramic, or plastic, with a wide mouth or opening that can be closed with a lid, screw cap, lug cap, cork stopper, roll-on cap, crimp-on cap, press-on cap, plastic shrink, heat sealed lidding film, an inner seal, a tamper-evident band, or other suitable means. The English word "jar" originates from the Arabic word jarra, which means an earthen pot or vessel.

A volumetric flask is a piece of laboratory apparatus, a type of laboratory flask, calibrated to contain a precise volume at a certain temperature. Volumetric flasks are used for precise dilutions and preparation of standard solutions. These flasks are usually pear-shaped, with a flat bottom, and made of glass or plastic. The flask's mouth is either furnished with a plastic snap/screw cap or fitted with a joint to accommodate a PTFE or glass stopper. The neck of volumetric flasks is elongated and narrow with an etched ring graduation marking. The marking indicates the volume of liquid contained when filled up to that point. The marking is typically calibrated "to contain" at 20 °C and indicated correspondingly on a label. The flask's label also indicates the nominal volume, tolerance, precision class, relevant manufacturing standard and the manufacturer's logo. Volumetric flasks are of various sizes, containing from a fraction of a milliliter to hundreds of liters of liquid.

A hot plate or hotplate is a portable self-contained tabletop small appliance cooktop that features one or more electric heating elements or gas burners. A hot plate can be used as a stand-alone appliance, but is often used as a substitute for one of the burners from an oven range or a kitchen stove. Hot plates are often used for food preparation, generally in locations where a full kitchen stove would not be convenient or practical. A hot plate can have a flat surface or round surface. Hot plates can be used for traveling or in areas without electricity.

Laboratory flasks are vessels or containers that fall into the category of laboratory equipment known as glassware. In laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes, but a common distinguishing aspect in their shapes is a wider vessel "body" and one narrower tubular sections at the top called necks which have an opening at the top. Laboratory flask sizes are specified by the volume they can hold, typically in metric units such as milliliters or liters. Laboratory flasks have traditionally been made of glass, but can also be made of plastic.

Rainer Ludwig Claisen was a German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn (1869), where he became a member of K.St.V. Arminia. He served in the army as a nurse in 1870–1871 and continued his studies at Göttingen University. He returned to the University of Bonn in 1872 and started his academic career at the same university in 1874. He died in 1930 in Godesberg am Rhein.
The Marcusson apparatus, Dean-Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water from a reactor. It is used in combination with a reflux condenser and a distillation flask for the separation of water from liquids. This may be a continuous removal of the water that is produced during a chemical reaction performed at reflux temperature, such as in esterification reactions. The original setup by Julius Marcusson was refined by the American chemists Ernest Woodward Dean (1888–1959) and David Dewey Stark (1893–1979) in 1920 for determination of the water content in petroleum.

A vial is a small glass or plastic vessel or bottle, often used to store medication in the form of liquids, powders, or capsules. They can also be used as scientific sample vessels; for instance, in autosampler devices in analytical chromatography. Vial-like glass containers date back to classical antiquity; modern vials are often made of plastics such as polypropylene. There are different types of vials such as a single dose vial and multi-dose vials often used for medications. The single dose vial is only used once whereas a multi-dose vial can be used more than once. The CDC sets specific guidelines on multi-dose vials.

Round-bottom flasks are types of flasks having spherical bottoms used as laboratory glassware, mostly for chemical or biochemical work. They are typically made of glass for chemical inertness; and in modern days, they are usually made of heat-resistant borosilicate glass. There is at least one tubular section known as the neck with an opening at the tip. Two- or three-necked flasks are common as well. Round bottom flasks come in many sizes, from 5 mL to 20 L, with the sizes usually inscribed on the glass. In pilot plants even larger flasks are encountered.
A Schlenk flask, or Schlenk tube, is a reaction vessel typically used in air-sensitive chemistry, invented by Wilhelm Schlenk. It has a side arm fitted with a PTFE or ground glass stopcock, which allows the vessel to be evacuated or filled with gases. These flasks are often connected to Schlenk lines, which allow both operations to be done easily.

Ground glass joints are used in laboratories to quickly and easily fit leak-tight apparatus together from interchangeable commonly available parts. For example, a round bottom flask, Liebig condenser, and oil bubbler with ground glass joints may be rapidly fitted together to reflux a reaction mixture. This is a large improvement compared with older methods of custom-made glassware, which was time-consuming and expensive, or the use of less chemical resistant and heat resistant corks or rubber bungs and glass tubes as joints, which took time to prepare as well.

Jebena is a traditional Ethiopian and Eritrean flask made of pottery and used to brew Arabic coffee. It is also widely used in Sudan, and the coffee itself is called bunna.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
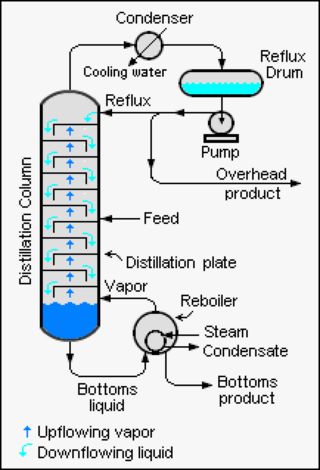
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.

Laboratory drying rack is a pegboard for hanging and draining glassware in a laboratory. It is available in different varieties and sizes. It can be used for different materials of glassware in the laboratory room such as funnels, pipettes, mixing balls, slides, bottle stoppers, tubing and so on. In addition to that, the pegs on the drying rack are easily removable and replaceable in order to maintain the cleaning of the lab racks to avoid contamination with other apparatus used on the same rack. Any common laboratory needs to have at least two or three drying racks per lab.


















