Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g. changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.

A dehumidifier is an air conditioning device which reduces and maintains the level of humidity in the air. This is done usually for health or thermal comfort reasons, or to eliminate musty odor and to prevent the growth of mildew by extracting water from the air. It can be used for household, commercial, or industrial applications. Large dehumidifiers are used in commercial buildings such as indoor ice rinks and swimming pools, as well as manufacturing plants or storage warehouses. Typical air conditioning systems combine dehumidification with cooling, by operating cooling coils below the dewpoint and draining away the water that condenses.
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate Na
2SiO
3, sodium orthosilicate Na
4SiO
4, and sodium pyrosilicate Na
6Si
2O
7. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.
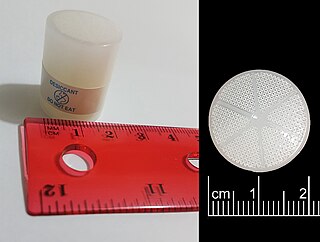
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams.

Desiccation is the state of extreme dryness, or the process of extreme drying. A desiccant is a hygroscopic substance that induces or sustains such a state in its local vicinity in a moderately sealed container. The word desiccation comes from Latin de- 'thoroughly', and siccare 'to dry'.

A glovebox is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hands into the gloves and perform tasks inside the box without breaking containment. Part or all of the box is usually transparent to allow the user to see what is being manipulated. Two types of gloveboxes exist. The first allows a person to work with hazardous substances, such as radioactive materials or infectious disease agents, and the second allows manipulation of substances that must be contained within a very high purity inert atmosphere, such as argon or nitrogen. It is also possible to use a glovebox for manipulation of items in a vacuum chamber.
A dry box is a storage container in which the interior is kept at a low level of humidity. It may be as simple as an airtight and watertight enclosure, or it may use active means to remove water vapor from the air trapped inside.

Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.

A molecular sieve is a material with pores of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrates through the stationary bed of porous, semi-solid substance referred to as a sieve, the components of the highest molecular weight leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in size-exclusion chromatography, a separation technique that sorts molecules based on their size. Another important use is as a desiccant. Most of molecular sieves are aluminosilicate zeolites with Si/Al molar ratio less than 2, but there are also examples of activated charcoal and silica gel.

A humidity indicator card (HIC) is a card on which a moisture-sensitive chemical is impregnated such that it will change color when the indicated relative humidity (RH) is exceeded. This has usually been a blotting paper impregnated with cobalt(II) chloride base; Less toxic alternatives include other chemicals such as cobalt-free chloride base and special plastic films.
An atmospheric water generator (AWG), is a device that extracts water from humid ambient air, producing potable water. Water vapor in the air can be extracted either by condensation - cooling the air below its dew point, exposing the air to desiccants, using membranes that only pass water vapor, collecting fog, or pressurizing the air. AWGs are useful where potable water is difficult to obtain, because water is always present in ambient air.

At equilibrium, the relationship between water content and equilibrium relative humidity of a material can be displayed graphically by a curve, the so-called moisture sorption isotherm. For each humidity value, a sorption isotherm indicates the corresponding water content value at a given, constant temperature. If the composition or quality of the material changes, then its sorption behaviour also changes. Because of the complexity of sorption process the isotherms cannot be determined explicitly by calculation, but must be recorded experimentally for each product.
Humidity buffering refers to the ability of materials to moderate changes in relative humidity by absorbing and desorbing water vapour from surrounding air. This is also referred to as moisture buffering.
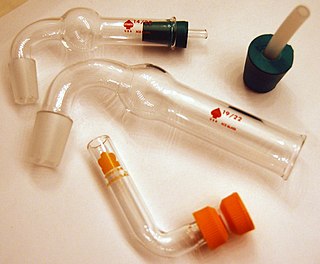
A drying tube or guard tube is a tube-like piece of apparatus used to house a disposable solid desiccant, wherein at one end the tube-like structure terminates in a ground glass joint for use in connecting the drying tube to a reaction vessel, for the purpose of keeping the vessel free of moisture.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.

Flower preservation has existed since early history, although deliberate flower preservation is a more recent phenomenon. In the Middle East, the bones of pre-historic man were discovered with delicate wild flowers probably as a tribute to a passing loved one. Evidence of deliberate use of specific flowers is indicated by the pollen grains that were present. Brightly colored and vivid flowers were also found in Egyptian tombs. These flowers were approximated to be 4,000 years old. In the sixteenth century medicinal nosegays began to give way to ornamental ones. Flowers essentially started to be used for decorative purposes such as jewels, fans and gloves. During the Elizabethan Age the once familiar ruff was replaced by soft lacy collars, and bosom flowers also became popular.

The Cromer cycle is a thermodynamic cycle that uses a desiccant to interact with higher relative humidity air leaving a cold surface. When a system is taken through a series of different states and finally returned to its initial state, a thermodynamic cycle is said to have occurred. The desiccant absorbs moisture from the air leaving the cold surface, releasing heat and drying the air, which can be used in a process requiring dry air. The desiccant is then dried by an air stream at a lower relative humidity, where the desiccant gives up its moisture by evaporation, increasing the air's relative humidity and cooling it. This cooler, moister air can then be presented to the same cold surface as above to take it below its dew point and dry it further, or it can be expunged from the system.












