
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products. Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.

Laboratory glassware refers to a variety of equipment used in scientific work, and traditionally made of glass. Glass can be blown, bent, cut, molded, and formed into many sizes and shapes, and is therefore common in chemistry, biology, and analytical laboratories. Many laboratories have training programs to demonstrate how glassware is used and to alert first–time users to the safety hazards involved with using glassware.

Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance.
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully.
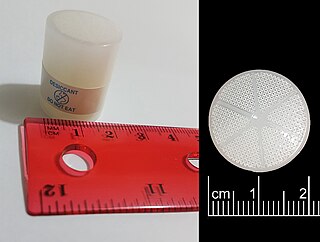
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams.

A diving air compressor is a gas compressor that can provide breathing air directly to a surface-supplied diver, or fill diving cylinders with high-pressure air pure enough to be used as a breathing gas. A low pressure diving air compressor usually has a delivery pressure of up to 30 bar, which is regulated to suit the depth of the dive. A high pressure diving compressor has a delivery pressure which is usually over 150 bar, and is commonly between 200 and 300 bar. The pressure is limited by an overpressure valve which may be adjustable.

Desiccators are sealable enclosures containing desiccants used for preserving moisture-sensitive items such as cobalt chloride paper for another use. A common use for desiccators is to protect chemicals which are hygroscopic or which react with water from humidity.

A molecular sieve is a material with pores of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrate through the stationary bed of porous, semi-solid substance referred to as a sieve, the components of highest molecular weight leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in size-exclusion chromatography, a separation technique that sorts molecules based on their size. Other molecular sieves are used as desiccants.
The Dean–Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water from a reactor. It is used in combination with a reflux condenser and a batch reactor for continuous removal of the water that is produced during a chemical reaction performed at reflux temperature. It was invented by the American chemists Ernest Woodward Dean (1888–1959) and David Dewey Stark (1893–1979) in 1920 for determination of the water content in petroleum.

The Schlenk line is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is connected to a vacuum pump. The inert-gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the vacuum pump by a liquid-nitrogen or dry-ice/acetone cold trap. Special stopcocks or Teflon taps allow vacuum or inert gas to be selected without the need for placing the sample on a separate line.
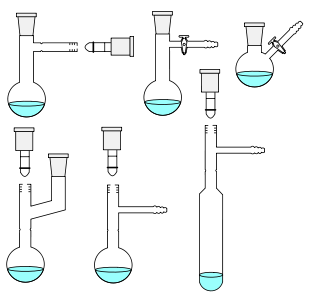
A Schlenk flask, or Schlenk tube, is a reaction vessel typically used in air-sensitive chemistry, invented by Wilhelm Schlenk. It has a side arm fitted with a PTFE or ground glass stopcock, which allows the vessel to be evacuated or filled with gases. These flasks are often connected to Schlenk lines, which allow both operations to be done easily.

A gas bubbler is a piece of laboratory glassware which consists of a glass bulb filled with a small amount of fluid — usually mineral or silicone oil, less commonly mercury. The inlet to the bulb is connected to a ground glass joint, while the outlet is vented to the air.

Ground glass joints are used in laboratories to quickly and easily fit leak-tight apparatus together from interchangeable commonly available parts. For example, a round bottom flask, Liebig condenser, and oil bubbler with ground glass joints may be rapidly fitted together to reflux a reaction mixture. This is a large improvement compared with older methods of custom-made glassware, which was time-consuming and expensive, or the use of less chemical resistant and heat resistant corks or rubber bungs and glass tubes as joints, which took time to prepare as well.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.

Cannula transfer or cannulation is a set of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. While the syringes are not the same as cannulae, the techniques remain relevant.
A Bubbler cylinder is a component of a unit for the metal organic chemical vapor deposition (MOCVD). They are devices that are used for conveying electronic grade metalorganic compounds from a liquid or solid precursor into a usable vapor.

Fritted glass is finely porous glass through which gas or liquid may pass. It is made by sintering together glass particles into a solid but porous body. This porous glass body can also be called a frit. Applications in laboratory glassware include use in fritted glass filter items, scrubbers, or spargers. Other laboratory applications of fritted glass include packing in chromatography columns and resin beds for special chemical synthesis.
Compressed air dryers are special types of filter systems that are specifically designed to remove the water that is inherent in compressed air. The process of compressing air raises its temperature and concentrates atmospheric contaminants, primarily water vapor. Consequently, the compressed air is generally at an elevated temperature and 100% relative humidity. As the compressed air cools, water vapor condenses into the tank(s), pipes, hoses and tools that are downstream from the compressor. Water vapor is removed from compressed air to prevent condensation from occurring and to prevent moisture from interfering in sensitive industrial processes.













