Cavitation in fluid mechanics and engineering normally refers to the phenomenon in which the static pressure of a liquid reduces to below the liquid's vapour pressure, leading to the formation of small vapor-filled cavities in the liquid. When subjected to higher pressure, these cavities, called "bubbles" or "voids", collapse and can generate shock waves that may damage machinery. These shock waves are strong when they are very close to the imploded bubble, but rapidly weaken as they propagate away from the implosion. Cavitation is a significant cause of wear in some engineering contexts. Collapsing voids that implode near to a metal surface cause cyclic stress through repeated implosion. This results in surface fatigue of the metal, causing a type of wear also called "cavitation". The most common examples of this kind of wear are to pump impellers, and bends where a sudden change in the direction of liquid occurs. Cavitation is usually divided into two classes of behavior: inertial cavitation and non-inertial cavitation.
An emulsion is a mixture of two or more liquids that are normally immiscible owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid is dispersed in the other. Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working.

Ultrasound is sound with frequencies greater than 20 kilohertz. This frequency is the approximate upper audible limit of human hearing in healthy young adults. The physical principles of acoustic waves apply to any frequency range, including ultrasound. Ultrasonic devices operate with frequencies from 20 kHz up to several gigahertz.

Sonoluminescence is the emission of light from imploding bubbles in a liquid when excited by sound.

Ultrasonic cleaning is a process that uses ultrasound to agitate a fluid, with a cleaning effect. Ultrasonic cleaners come in a variety of sizes, from small desktop units with an internal volume of less than 0.5 litres (0.13 US gal), to large industrial units with volumes approaching 1,000 litres.
In chemistry, the study of sonochemistry is concerned with understanding the effect of ultrasound in forming acoustic cavitation in liquids, resulting in the initiation or enhancement of the chemical activity in the solution. Therefore, the chemical effects of ultrasound do not come from a direct interaction of the ultrasonic sound wave with the molecules in the solution.

High-intensity focused ultrasound (HIFU), or MR-guided Focused Ultrasound Surgery is an incision-less therapeutic technique that uses non-ionizing ultrasonic waves to heat or ablate tissue. HIFU can be used to increase the flow of blood or lymph or to destroy tissue, such as tumors, via thermal and mechanical mechanisms. Given the prevalence and relatively low cost of ultrasound generation mechanisms, the premise of HIFU is that it is expected to be a non-invasive and low-cost therapy that can at least outperform care in the operating room.
The ASA Silver Medal is an award presented by the Acoustical Society of America to individuals, without age limitation, for contributions to the advancement of science, engineering, or human welfare through the application of acoustic principles or through research accomplishments in acoustics. The medal is awarded in a number of categories depending on the technical committee responsible for making the nomination.

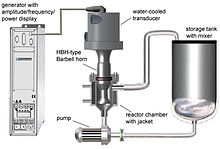
An ultrasonic horn is a tapering metal bar commonly used for augmenting the oscillation displacement amplitude provided by an ultrasonic transducer operating at the low end of the ultrasonic frequency spectrum. The device is necessary because the amplitudes provided by the transducers themselves are insufficient for most practical applications of power ultrasound. Another function of the ultrasonic horn is to efficiently transfer the acoustic energy from the ultrasonic transducer into the treated media, which may be solid or liquid. Ultrasonic processing of liquids relies of intense shear forces and extreme local conditions generated by acoustic cavitation.
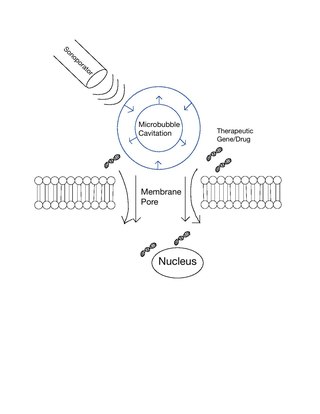
Sonoporation, or cellular sonication, is the use of sound in the ultrasonic range for increasing the permeability of the cell plasma membrane. This technique is usually used in molecular biology and non-viral gene therapy in order to allow uptake of large molecules such as DNA into the cell, in a cell disruption process called transfection or transformation. Sonoporation employs the acoustic cavitation of microbubbles to enhance delivery of these large molecules. The exact mechanism of sonoporation-mediated membrane translocation remains unclear, with a few different hypotheses currently being explored.

Kenneth S. Suslick is the Marvin T. Schmidt Professor of Chemistry Emeritus at the University of Illinois at Urbana–Champaign. His area of focus is on the chemical and physical effects of ultrasound, sonochemistry, and sonoluminescence. In addition, he has worked in the fields of artificial and machine olfaction, electronic nose technology, chemical sensor arrays, and the use of colorimetric sensor arrays as an optoelectronic nose.
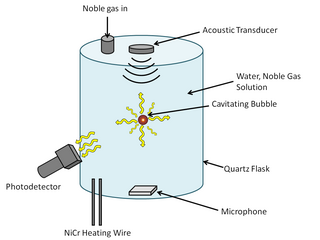
Sonoluminescence is a phenomenon that occurs when a small gas bubble is acoustically suspended and periodically driven in a liquid solution at ultrasonic frequencies, resulting in bubble collapse, cavitation, and light emission. The thermal energy that is released from the bubble collapse is so great that it can cause weak light emission. The mechanism of the light emission remains uncertain, but some of the current theories, which are categorized under either thermal or electrical processes, are Bremsstrahlung radiation, argon rectification hypothesis, and hot spot. Some researchers are beginning to favor thermal process explanations as temperature differences have consistently been observed with different methods of spectral analysis. In order to understand the light emission mechanism, it is important to know what is happening in the bubble's interior and at the bubble's surface.

Sonodynamic therapy (SDT) is a noninvasive treatment, often used for tumor irradiation, that utilizes a sonosensitizer and the deep penetration of ultrasound to treat lesions of varying depths by reducing target cell number and preventing future tumor growth. Many existing cancer treatment strategies cause systemic toxicity or cannot penetrate tissue deep enough to reach the entire tumor; however, emerging ultrasound stimulated therapies could offer an alternative to these treatments with their increased efficiency, greater penetration depth, and reduced side effects. Sonodynamic therapy could be used to treat cancers and other diseases, such as atherosclerosis, and diminish the risk associated with other treatment strategies since it induces cytotoxic effects only when externally stimulated by ultrasound and only at the cancerous region, as opposed to the systemic administration of chemotherapy drugs.

Timothy Grant Leighton is a British scientist who was a Professor of Ultrasonics and Underwater Acoustics at the University of Southampton. He is the inventor-in-chief of Sloan Water Technology Ltd., a company founded around his inventions. He is an academician of three national academies. Trained in physics and theoretical physics, he works across physical, medical, biological, social and ocean sciences, fluid dynamics and engineering. He joined the Institute of Sound and Vibration Research (ISVR) at the University of Southampton in 1992 as a lecturer in underwater acoustics, and completed the monograph The Acoustic Bubble in the same year. He was awarded a personal chair at the age of 35 and has authored over 400 publications.
Ultrasonic antifouling is a technology that uses high frequency sound (ultrasound) to prevent or reduce biofouling on underwater structures, surfaces, and medium. Ultrasound is just high frequency sound. Ultrasound has the same physical properties as human-audible sound. The method has two primary forms: sub-cavitation intensity and cavitation intensity. Sub-cavitation methods create high frequency vibrations, whilst cavitation methods cause more destructive microscopic pressure changes. Both methods inhibit or prevent biofouling by algae and other single-celled organisms.
Sonoelectrochemistry is the application of ultrasound in electrochemistry. Like sonochemistry, sonoelectrochemistry was discovered in the early 20th century. The effects of power ultrasound on electrochemical systems and important electrochemical parameters were originally demonstrated by Moriguchi and then by Schmid and Ehert when the researchers investigated the influence of ultrasound on concentration polarisation, metal passivation and the production of electrolytic gases in aqueous solutions. In the late 1950s, Kolb and Nyborg showed that the electrochemical solution hydrodynamics in an electrochemical cell was greatly increased in the presence of ultrasound and described this phenomenon as acoustic streaming. In 1959, Penn et al. demonstrated that sonication had a great effect on the electrode surface activity and electroanalyte species concentration profile throughout the solution. In the early 1960s, the electrochemist Allen J. Bard showed in controlled potential coulometry experiments that ultrasound significantly enhances mass transport of electrochemical species from the bulk solution to the electroactive surface. In the range of ultrasonic frequencies [20 kHz – 2 MHz], ultrasound has been applied to many electrochemical systems, processes and areas of electrochemistry both in academia and industry, as this technology offers several benefits over traditional technologies. The advantages are as follows: significant thinning of the diffusion layer thickness (δ) at the electrode surface; increase in electrodeposit/electroplating thickness; increase in electrochemical rates, yields and efficiencies; increase in electrodeposit porosity and hardness; increase in gas removal from electrochemical solutions; increase in electrode cleanliness and hence electrode surface activation; lowering in electrode overpotentials ; and suppression in electrode fouling.
Sonochemical synthesis is the process which utilizes the principles of sonochemistry to make molecules undergo a chemical reaction with the application of powerful ultrasound radiation (20 kHz–10 MHz). Sonochemistry generates hot spots that can achieve very high temperatures, pressures of more than 1000 atmospheres, and rates of heating and cooling that can exceed 10^11 K/s. High intensity ultrasound produces chemical and physical effects that can be used for the production or modification of a wide range of nanostructured materials. The principle that causes the modification of nanostructures in the sonochemical process is acoustic cavitation.

Aniruddha Bhalchandra Pandit (born 7 December 1957) is an Indian chemical engineer, inventor and academic, known for his fundamental and commercial research on cavitational reactors, design of multiphase reactors, bubble dynamics. He is the vice chancellor of the Institute of Chemical Technology, Mumbai since 2019, succeeding G. D. Yadav.
Ultrasound-triggered drug delivery using stimuli-responsive hydrogels refers to the process of using ultrasound energy for inducing drug release from hydrogels that are sensitive to acoustic stimuli. This method of approach is one of many stimuli-responsive drug delivery-based systems that has gained traction in recent years due to its demonstration of localization and specificity of disease treatment. Although recent developments in this field highlight its potential in treating certain diseases such as COVID-19, there remain many major challenges that need to be addressed and overcome before more related biomedical applications are clinically translated into standard of care.
Sonocatalysis is a field of sonochemistry which is based on the use of ultrasound to change the reactivity of a catalyst in homogenous or heterogenous catalysis. It is generally used to support catalysis. This method of catalysis has been known since the creation of sonochemistry in 1927 by Alfred Lee Loomis (1887–1975) and Robert Williams Wood (1868–1955). Sonocatalysis depends on ultrasounds, which were discovered in 1794 by the Italian biologist Lazarro Spallanzani (1729–1799).