
Electroporation, or electropermeabilization, is a technique in which an electrical field is applied to cells in order to increase the permeability of the cell membrane. This may allow chemicals, drugs, electrode arrays or DNA to be introduced into the cell.
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small fragments of nucleic acids can be manufactured as single-stranded molecules with any user-specified sequence, and so are vital for artificial gene synthesis, polymerase chain reaction (PCR), DNA sequencing, molecular cloning and as molecular probes. In nature, oligonucleotides are usually found as small RNA molecules that function in the regulation of gene expression, or are degradation intermediates derived from the breakdown of larger nucleic acid molecules.

Small interfering RNA (siRNA), sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded non-coding RNA molecules, typically 20–24 base pairs in length, similar to microRNA (miRNA), and operating within the RNA interference (RNAi) pathway. It interferes with the expression of specific genes with complementary nucleotide sequences by degrading messenger RNA (mRNA) after transcription, preventing translation. It was discovered in 1998 by Andrew Fire at the Carnegie Institution for Science in Washington, D.C. and Craig Mello at the University of Massachusetts in Worcester.
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. It may also refer to other methods and cell types, although other terms are often preferred: "transformation" is typically used to describe non-viral DNA transfer in bacteria and non-animal eukaryotic cells, including plant cells. In animal cells, transfection is the preferred term as transformation is also used to refer to progression to a cancerous state (carcinogenesis) in these cells. Transduction is often used to describe virus-mediated gene transfer into eukaryotic cells.
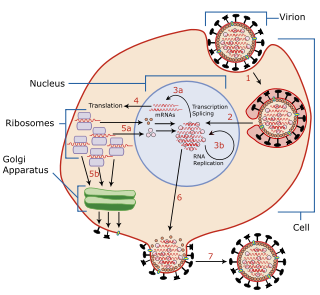
Viral replication is the formation of biological viruses during the infection process in the target host cells. Viruses must first get into the cell before viral replication can occur. Through the generation of abundant copies of its genome and packaging these copies, the virus continues infecting new hosts. Replication between viruses is greatly varied and depends on the type of genes involved in them. Most DNA viruses assemble in the nucleus while most RNA viruses develop solely in cytoplasm.

Contrast-enhanced ultrasound (CEUS) is the application of ultrasound contrast medium to traditional medical sonography. Ultrasound contrast agents rely on the different ways in which sound waves are reflected from interfaces between substances. This may be the surface of a small air bubble or a more complex structure. Commercially available contrast media are gas-filled microbubbles that are administered intravenously to the systemic circulation. Microbubbles have a high degree of echogenicity. There is a great difference in echogenicity between the gas in the microbubbles and the soft tissue surroundings of the body. Thus, ultrasonic imaging using microbubble contrast agents enhances the ultrasound backscatter, (reflection) of the ultrasound waves, to produce a sonogram with increased contrast due to the high echogenicity difference. Contrast-enhanced ultrasound can be used to image blood perfusion in organs, measure blood flow rate in the heart and other organs, and for other applications.

Cationic liposomes are spherical structures that contain positively charged lipids. Cationic liposomes can vary in size between 40 nm and 500 nm, and they can either have one lipid bilayer (monolamellar) or multiple lipid bilayers (multilamellar). The positive charge of the phospholipids allows cationic liposomes to form complexes with negatively charged nucleic acids through ionic interactions. Upon interacting with nucleic acids, cationic liposomes form clusters of aggregated vesicles. These interactions allow cationic liposomes to condense and encapsulate various therapeutic and diagnostic agents in their aqueous compartment or in their lipid bilayer. These cationic liposome-nucleic acid complexes are also referred to as lipoplexes. Due to the overall positive charge of cationic liposomes, they interact with negatively charged cell membranes more readily than classic liposomes. This positive charge can also create some issues in vivo, such as binding to plasma proteins in the bloodstream, which leads to opsonization. These issues can be reduced by optimizing the physical and chemical properties of cationic liposomes through their lipid composition. Cationic liposomes are increasingly being researched for use as delivery vectors in gene therapy due to their capability to efficiently transfect cells. A common application for cationic liposomes is cancer drug delivery.

Sonophoresis also known as phonophoresis, is a method that utilizes ultrasound to enhance the delivery of topical medications through the stratum corneum, to the epidermis and dermis. Sonophoresis allows for the enhancement of the permeability of the skin along with other modalities, such as iontophoresis, to deliver drugs with lesser side effects. Currently, sonophoresis is used widely in transdermal drug delivery, but has potential applications in other sectors of drug delivery, such as the delivery of drugs to the eye and brain.

Gene delivery is the process of introducing foreign genetic material, such as DNA or RNA, into host cells. Gene delivery must reach the genome of the host cell to induce gene expression. Successful gene delivery requires the foreign gene delivery to remain stable within the host cell and can either integrate into the genome or replicate independently of it. This requires foreign DNA to be synthesized as part of a vector, which is designed to enter the desired host cell and deliver the transgene to that cell's genome. Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors.
Cell-penetrating peptides (CPPs) are short peptides that facilitate cellular intake and uptake of molecules ranging from nanosize particles to small chemical compounds to large fragments of DNA. The "cargo" is associated with the peptides either through chemical linkage via covalent bonds or through non-covalent interactions.
Vesicle fusion is the merging of a vesicle with other vesicles or a part of a cell membrane. In the latter case, it is the end stage of secretion from secretory vesicles, where their contents are expelled from the cell through exocytosis. Vesicles can also fuse with other target cell compartments, such as a lysosome. Exocytosis occurs when secretory vesicles transiently dock and fuse at the base of cup-shaped structures at the cell plasma membrane called porosome, the universal secretory machinery in cells. Vesicle fusion may depend on SNARE proteins in the presence of increased intracellular calcium (Ca2+) concentration.
Optical transfection is a biomedical technique that entails introducing nucleic acids into cells using light. All cells are surrounded by a plasma membrane, which prevents many substances from entering or exiting the cell. Lasers can be used to burn a tiny hole in this membrane, allowing substances to enter. This is tremendously useful to biologists who are studying disease, as a common experimental requirement is to put things into cells.
Microbubbles are bubbles smaller than one hundredth of a millimetre in diameter, but larger than one micrometre. They have widespread application in industry, medicine, life science, and food technology. The composition of the bubble shell and filling material determine important design features such as buoyancy, crush strength, thermal conductivity, and acoustic properties.

Gene therapy utilizes the delivery of DNA into cells, which can be accomplished by several methods, summarized below. The two major classes of methods are those that use recombinant viruses and those that use naked DNA or DNA complexes.

Sonodynamic therapy (SDT) is a noninvasive treatment, often used for tumor irradiation, that utilizes a sonosensitizer and the deep penetration of ultrasound to treat lesions of varying depths by reducing target cell number and preventing future tumor growth. Many existing cancer treatment strategies cause systemic toxicity or cannot penetrate tissue deep enough to reach the entire tumor; however, emerging ultrasound stimulated therapies could offer an alternative to these treatments with their increased efficiency, greater penetration depth, and reduced side effects. Sonodynamic therapy could be used to treat cancers and other diseases, such as atherosclerosis, and diminish the risk associated with other treatment strategies since it induces cytotoxic effects only when externally stimulated by ultrasound and only at the cancerous region, as opposed to the systemic administration of chemotherapy drugs.
Nanoparticles for drug delivery to the brain is a method for transporting drug molecules across the blood–brain barrier (BBB) using nanoparticles. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson's disease, Alzheimer's disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, antineoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone. With the aid of nanoparticle delivery systems, however, studies have shown that some drugs can now cross the BBB, and even exhibit lower toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function. Further, the BBB is not the only physiological barrier for drug delivery to the brain. Other biological factors influence how drugs are transported throughout the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis. Though there exist many obstacles that make developing a robust delivery system difficult, nanoparticles provide a promising mechanism for drug transport to the CNS.
Poly(amidoamine), or PAMAM, is a class of dendrimer which is made of repetitively branched subunits of amide and amine functionality. PAMAM dendrimers, sometimes referred to by the trade name Starburst, have been extensively studied since their synthesis in 1985, and represent the most well-characterized dendrimer family as well as the first to be commercialized. Like other dendrimers, PAMAMs have a sphere-like shape overall, and are typified by an internal molecular architecture consisting of tree-like branching, with each outward 'layer', or generation, containing exponentially more branching points. This branched architecture distinguishes PAMAMs and other dendrimers from traditional polymers, as it allows for low polydispersity and a high level of structural control during synthesis, and gives rise to a large number of surface sites relative to the total molecular volume. Moreover, PAMAM dendrimers exhibit greater biocompatibility than other dendrimer families, perhaps due to the combination of surface amines and interior amide bonds; these bonding motifs are highly reminiscent of innate biological chemistry and endow PAMAM dendrimers with properties similar to that of globular proteins. The relative ease/low cost of synthesis of PAMAM dendrimers (especially relative to similarly-sized biological molecules such as proteins and antibodies), along with their biocompatibility, structural control, and functionalizability, have made PAMAMs viable candidates for application in drug development, biochemistry, and nanotechnology.

Focused ultrasound for intracrainial drug delivery is a non-invasive technique that uses high-frequency sound waves to disrupt tight junctions in the blood–brain barrier (BBB), allowing for increased passage of therapeutics into the brain. The BBB normally blocks nearly 98% of drugs from accessing the central nervous system, so FUS has the potential to address a major challenge in intracranial drug delivery by providing targeted and reversible BBB disruption. Using FUS to enhance drug delivery to the brain could significantly improve patient outcomes for a variety of diseases including Alzheimer's disease, Parkinson's disease, and brain cancer.
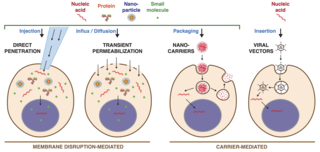
Intracellular delivery is the process of introducing external materials into living cells. Materials that are delivered into cells include nucleic acids, proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects. Such molecules and materials can be used to investigate cellular behavior, engineer cell operations or correct a pathological function.
Focused-ultrasound-mediated diagnostics or FUS-mediated diagnostics are an area of clinical diagnostic tools that use ultrasound to detect diseases and cancers. Although ultrasound has been used for imaging in various settings, focused-ultrasound refers to the detection of specific cells and biomarkers under flow combining ultrasound with lasers, microbubbles, and imaging techniques. Current diagnostic techniques for detecting tumors and diseases using biopsies often include invasive procedures and require improved accuracy, especially in cases such as glioblastoma and melanoma. The field of FUS-mediated diagnostics targeting cells and biomarkers is being investigated for overcoming these limitations.













