
Intracytoplasmic sperm injection is an in vitro fertilization (IVF) procedure in which a single sperm cell is injected directly into the cytoplasm of an egg. This technique is used in order to prepare the gametes for the obtention of embryos that may be transferred to a maternal uterus. With this method, the acrosome reaction is skipped.

For fertilization to happen between a sperm and egg cell, a sperm must first fuse with the plasma membrane and then penetrate the female egg cell to fertilize it. While the fusion of the sperm cell with the egg cell's plasma membrane is relatively straightforward, penetrating the egg's protective layers, such as the zona pellucida, presents a significant challenge. Therefore, sperm cells go through a process known as the acrosome reaction, which is the reaction that occurs in the acrosome of the sperm as it approaches the egg.

Nuclear transfer is a form of cloning. The step involves removing the DNA from an oocyte, and injecting the nucleus which contains the DNA to be cloned. In rare instances, the newly constructed cell will divide normally, replicating the new DNA while remaining in a pluripotent state. If the cloned cells are placed in the uterus of a female mammal, a cloned organism develops to term in rare instances. This is how Dolly the Sheep and many other species were cloned. Cows are commonly cloned to select those that have the best milk production. On 24 January 2018, two monkey clones were reported to have been created with the technique for the first time.
Polly and Molly, two ewes, were the first mammals to have been successfully cloned from an adult somatic cell and to be transgenic animals at the same time. This is not to be confused with Dolly the Sheep, the first animal to be successfully cloned from an adult somatic cell where there wasn’t modification carried out on the adult donor nucleus. Polly and Molly, like Dolly the Sheep, were cloned at the Roslin Institute in Edinburgh, Scotland.
A transgene is a gene that has been transferred naturally, or by any of a number of genetic engineering techniques, from one organism to another. The introduction of a transgene, in a process known as transgenesis, has the potential to change the phenotype of an organism. Transgene describes a segment of DNA containing a gene sequence that has been isolated from one organism and is introduced into a different organism. This non-native segment of DNA may either retain the ability to produce RNA or protein in the transgenic organism or alter the normal function of the transgenic organism's genetic code. In general, the DNA is incorporated into the organism's germ line. For example, in higher vertebrates this can be accomplished by injecting the foreign DNA into the nucleus of a fertilized ovum. This technique is routinely used to introduce human disease genes or other genes of interest into strains of laboratory mice to study the function or pathology involved with that particular gene.

Exogenous DNA is DNA originating outside the organism of concern or study. Exogenous DNA can be found naturally in the form of partially degraded fragments left over from dead cells. These DNA fragments may then become integrated into the chromosomes of nearby bacterial cells to undergo mutagenesis. This process of altering bacteria is known as transformation. Bacteria may also undergo artificial transformation through chemical and biological processes. The introduction of exogenous DNA into eukaryotic cells is known as transfection. Exogenous DNA can also be artificially inserted into the genome, which revolutionized the process of genetic modification in animals. By microinjecting an artificial transgene into the nucleus of an animal embryo, the exogenous DNA is allowed to merge the cell's existing DNA to create a genetically modified, transgenic animal. The creation of transgenic animals also leads into the study of altering sperm cells with exogenous DNA.

Ralph Lawrence Brinster is an American geneticist, National Medal of Science laureate, and Richard King Mellon Professor of Reproductive Physiology at the School of Veterinary Medicine, University of Pennsylvania.
Sperm-mediated gene transfer (SMGT) is a transgenic technique that transfers genes based on the ability of sperm cells to spontaneously bind to and internalize exogenous DNA and transport it into an oocyte during fertilization to produce genetically modified animals.1 Exogenous DNA refers to DNA that originates outside of the organism. Transgenic animals have been obtained using SMGT, but the efficiency of this technique is low. Low efficiency is mainly due to low uptake of exogenous DNA by the spermatozoa, reducing the chances of fertilizing the oocytes with transfected spermatozoa.2 In order to successfully produce transgenic animals by SMGT, the spermatozoa must attach the exogenous DNA into the head and these transfected spermatozoa must maintain their functionality to fertilize the oocyte.2 Genetically modified animals produced by SMGT are useful for research in biomedical, agricultural, and veterinary fields of study. SMGT could also be useful in generating animals as models for human diseases or lead to future discoveries relating to human gene therapy.

A micromanipulator is a device which is used to physically interact with a sample under a microscope, where a level of precision of movement is necessary that cannot be achieved by the unaided human hand. It may typically consist of an input joystick, a mechanism for reducing the range of movement and an output section with the means of holding a microtool to hold, inject, cut or otherwise manipulate the object as required. The mechanism for reducing the movement usually requires the movement to be free of backlash. This is achieved by the use of kinematic constraints to allow each part of the mechanism to move only in one or more chosen degrees of freedom, which achieves a high precision and repeatability of movement, usually at the expense of some absolute accuracy.

A genetically modified mouse or genetically engineered mouse model (GEMM) is a mouse that has had its genome altered through the use of genetic engineering techniques. Genetically modified mice are commonly used for research or as animal models of human diseases and are also used for research on genes. Together with patient-derived xenografts (PDXs), GEMMs are the most common in vivo models in cancer research. Both approaches are considered complementary and may be used to recapitulate different aspects of disease. GEMMs are also of great interest for drug development, as they facilitate target validation and the study of response, resistance, toxicity and pharmacodynamics.

A knockout rat is a genetically engineered rat with a single gene turned off through a targeted mutation used for academic and pharmaceutical research. Knockout rats can mimic human diseases and are important tools for studying gene function and for drug discovery and development. The production of knockout rats was not economically or technically feasible until 2008.
The Genetics & IVF Institute (GIVF) is an international provider of infertility and genetics services and products, and also engages in biomedical research in these fields. The Institute was founded in 1984 by Dr. Joseph D. Schulman and associates. GIVF headquarters are in Fairfax, VA, US, and its facilities include locations in Pennsylvania, Minnesota, California, and Texas in the United States, as well as in China, Mexico, and several other countries.
Teratospermia or teratozoospermia is a condition characterized by the presence of sperm with abnormal morphology that affects fertility in males.
Oocyteactivation is a series of processes that occur in the oocyte during fertilization.
Nanoinjection is the process of using a microscopic lance and electrical forces to deliver DNA to a cell. It is claimed to be more effective than microinjection because the lance used is ten times smaller than a micropipette and the method uses no fluid. The nanoinjector mechanism is operated while submerged in a pH buffered solution. Then, a positive electrical charge is applied to the lance, which accumulates negatively charged DNA on its surface. The nanoinjector mechanism then penetrates the zygotic membranes, and a negative charge is applied to the lance, releasing the accumulated DNA within the cell. The lance is required to maintain a constant elevation on both entry and exit of the cell.
Breast cancer metastatic mouse models are experimental approaches in which mice are genetically manipulated to develop a mammary tumor leading to distant focal lesions of mammary epithelium created by metastasis. Mammary cancers in mice can be caused by genetic mutations that have been identified in human cancer. This means models can be generated based upon molecular lesions consistent with the human disease.
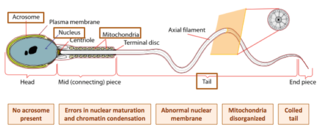
Globozoospermia is a rare and severe form of monomorphic teratozoospermia. This means that the spermatozoa show the same abnormality, and over 85% of spermatozoa in sperm have this abnormality. Globozoospermia is responsible for less than 0.1% of male infertility. It is characterised by round-headed spermatozoa without acrosomes, an abnormal nuclear membrane and midpiece defects. Affected males therefore suffer from either reduced fertility or infertility. Studies suggest that globozoospermia can be either total or partial, however it is unclear whether these two forms are variations on the same syndrome, or actually different syndromes.

The intracytoplasmic morphologically selected sperm injection (IMSI) is a laboratory technique used for In vitro fertilisation treatments. High-quality sperms are injected into the egg for fertilization, it is an advanced version of ICSI.
Dmitri Dozortsev is a Russian-American physician scientist, inventor and researcher. Dozortsev's contributions in research and publications are mostly in the areas of human reproductive medicine and biology. In particular, he is best known for his studies of in vitro fertilisation and embryo transfer. Dozortsev currently serves as President of the American College of Embryology and as Director of Omni-Med laboratories.
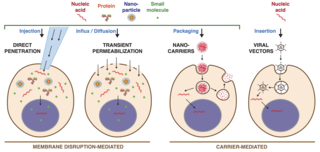
Intracellular delivery is the process of introducing external materials into living cells. Materials that are delivered into cells include nucleic acids, proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects. Such molecules and materials can be used to investigate cellular behavior, engineer cell operations or correct a pathological function.











