Stem cell research
Somatic cell nuclear transplantation has become a focus of study in stem cell research. The aim of carrying out this procedure is to obtain pluripotent cells from a cloned embryo. These cells genetically matched the donor organism from which they came. This gives them the ability to create patient specific pluripotent cells, which could then be used in therapies or disease research. [13]
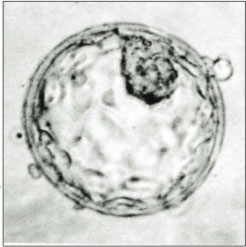
Embryonic stem cells are undifferentiated cells of an embryo. These cells are deemed to have a pluripotent potential because they have the ability to give rise to all of the tissues found in an adult organism. This ability allows stem cells to create any cell type, which could then be transplanted to replace damaged or destroyed cells. Controversy surrounds human ESC work due to the destruction of viable human embryos, leading scientists to seek alternative methods of obtaining pluripotent stem cells, SCNT is one such method.
A potential use of stem cells genetically matched to a patient would be to create cell lines that have genes linked to a patient's particular disease. By doing so, an in vitro model could be created, would be useful for studying that particular disease, potentially discovering its pathophysiology, and discovering therapies. [14] For example, if a person with Parkinson's disease donated their somatic cells, the stem cells resulting from SCNT would have genes that contribute to Parkinson's disease. The disease specific stem cell lines could then be studied in order to better understand the condition. [15]
Another application of SCNT stem cell research is using the patient specific stem cell lines to generate tissues or even organs for transplant into the specific patient. [16] The resulting cells would be genetically identical to the somatic cell donor, thus avoiding any complications from immune system rejection. [15] [17]
Only a handful of the labs in the world are currently using SCNT techniques in human stem cell research. In the United States, scientists at the Harvard Stem Cell Institute, the University of California San Francisco, the Oregon Health & Science University, [18] Stemagen (La Jolla, CA) and possibly Advanced Cell Technology are currently researching a technique to use somatic cell nuclear transfer to produce embryonic stem cells. [19] In the United Kingdom, the Human Fertilisation and Embryology Authority has granted permission to research groups at the Roslin Institute and the Newcastle Centre for Life. [20] SCNT may also be occurring in China. [21]
Though there has been numerous successes with cloning animals, questions remain concerning the mechanisms of reprogramming in the ovum. Despite many attempts, success in creating human nuclear transfer embryonic stem cells has been limited. There lies a problem in the human cell's ability to form a blastocyst; the cells fail to progress past the eight cell stage of development. This is thought to be a result from the somatic cell nucleus being unable to turn on embryonic genes crucial for proper development. These earlier experiments used procedures developed in non-primate animals with little success.
A research group from the Oregon Health & Science University demonstrated SCNT procedures developed for primates successfully using skin cells. The key to their success was utilizing oocytes in metaphase II (MII) of the cell cycle. Egg cells in MII contain special factors in the cytoplasm that have a special ability in reprogramming implanted somatic cell nuclei into cells with pluripotent states. When the ovum's nucleus is removed, the cell loses its genetic information. This has been blamed for why enucleated eggs are hampered in their reprogramming ability. It is theorized the critical embryonic genes are physically linked to oocyte chromosomes, enucleation negatively affects these factors. Another possibility is removing the egg nucleus or inserting the somatic nucleus causes damage to the cytoplast, affecting reprogramming ability.
Taking this into account the research group applied their new technique in an attempt to produce human SCNT stem cells. In May 2013, the Oregon group reported the successful derivation of human embryonic stem cell lines derived through SCNT, using fetal and infant donor cells. Using MII oocytes from volunteers and their improved SCNT procedure, human clone embryos were successfully produced. These embryos were of poor quality, lacking a substantial inner cell mass and poorly constructed trophectoderm. The imperfect embryos prevented the acquisition of human ESC. The addition of caffeine during the removal of the ovum's nucleus and fusion of the somatic cell and the egg improved blastocyst formation and ESC isolation. The ESC obtain were found to be capable of producing teratomas, expressed pluripotent transcription factors, and expressed a normal 46XX karyotype, indicating these SCNT were in fact ESC-like. [18] This was the first instance of successfully using SCNT to reprogram human somatic cells. This study used fetal and infantile somatic cells to produce their ESC.
In April 2014, an international research team expanded on this break through. There remained the question of whether the same success could be accomplished using adult somatic cells. Epigenetic and age related changes were thought to possibly hinder an adult somatic cells ability to be reprogrammed. Implementing the procedure pioneered by the Oregon research group they indeed were able to grow stem cells generated by SCNT using adult cells from two donors aged 35 and 75, indicating that age does not impede a cell's ability to be reprogrammed. [22] [23]
Late April 2014, the New York Stem Cell Foundation was successful in creating SCNT stem cells derived from adult somatic cells. One of these lines of stem cells was derived from the donor cells of a type 1 diabetic. The group was then able to successfully culture these stem cells and induce differentiation. When injected into mice, cells of all three of the germ layers successfully formed. The most significant of these cells, were those who expressed insulin and were capable of secreting the hormone. [24] These insulin producing cells could be used for replacement therapy in diabetics, demonstrating real SCNT stem cell therapeutic potential.
The impetus for SCNT-based stem cell research has been decreased by the development and improvement of alternative methods of generating stem cells. Methods to reprogram normal body cells into pluripotent stem cells were developed in humans in 2007. The following year, this method achieved a key goal of SCNT-based stem cell research: the derivation of pluripotent stem cell lines that have all genes linked to various diseases. [25] Some scientists working on SCNT-based stem cell research have recently moved to the new methods of induced pluripotent stem cells. Though recent studies have put in question how similar iPS cells are to embryonic stem cells. Epigenetic memory in iPS affects the cell lineage it can differentiate into. For instance, an iPS cell derived from a blood cell using only the yamanaka factors will be more efficient at differentiating into blood cells, while it will be less efficient at creating a neuron. [26] Recent studies indicate however that changes to the epigenetic memory of iPSCs using small molecules can reset them to an almost naive state of pluripotency. [27] [28] Studies have even shown that via tetraploid complementation, an entire viable organism can be created solely from iPSCs. [29] SCNT stem cells have been found to have similar challenges. The cause for low yields in bovine SCNT cloning has, in recent years, been attributed to the previously hidden epigenetic memory of the somatic cells that were being introduced into the oocyte. [30]
Reproductive cloning
This technique is currently the basis for cloning animals (such as the famous Dolly the sheep), [31] and has been proposed as a possible way to clone humans. Using SCNT in reproductive cloning has proven difficult with limited success. High fetal and neonatal death make the process very inefficient. Resulting cloned offspring are also plagued with development and imprinting disorders in non-human species. For these reasons, along with moral and ethical objections, reproductive cloning in humans is proscribed in more than 30 countries. [32] Most researchers believe that in the foreseeable future it will not be possible to use the current cloning technique to produce a human clone that will develop to term. It remains a possibility, though critical adjustments will be required to overcome current limitations during early embryonic development in human SCNT. [33] [34]
There is also the potential for treating diseases associated with mutations in mitochondrial DNA. Recent studies show SCNT of the nucleus of a body cell afflicted with one of these diseases into a healthy oocyte prevents the inheritance of the mitochondrial disease. This treatment does not involve cloning but would produce a child with three genetic parents. A father providing a sperm cell, one mother providing the egg nucleus, and another mother providing the enucleated egg cell. [16]
In 2018, the first successful cloning of primates using somatic cell nuclear transfer, the same method as Dolly the sheep, with the birth of two live female clones (crab-eating macaques named Zhong Zhong and Hua Hua) was reported. [3] [35] [36] [37] [38]