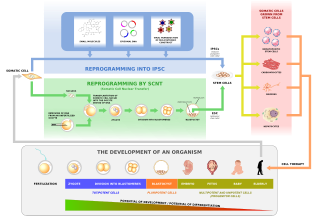
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissue. It does not refer to the natural conception and delivery of identical twins. The possibilities of human cloning have raised controversies. These ethical concerns have prompted several nations to pass laws regarding human cloning.

In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.

In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an denucleated oocyte and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal. In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques from foetal nuclei.

Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

Homeobox protein NANOG(hNanog) is a transcriptional factor that helps embryonic stem cells (ESCs) maintain pluripotency by suppressing cell determination factors. hNanog is encoded in humans by the NANOG gene. Several types of cancer are associated with NANOG.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced stem cells. They are commonly used in research and regenerative medicine.
The stem cell controversy is the consideration of the ethics of research involving the development and use of human embryos. Most commonly, this controversy focuses on embryonic stem cells. Not all stem cell research involves human embryos. For example, adult stem cells, amniotic stem cells, and induced pluripotent stem cells do not involve creating, using, or destroying human embryos, and thus are minimally, if at all, controversial. Many less controversial sources of acquiring stem cells include using cells from the umbilical cord, breast milk, and bone marrow, which are not pluripotent.

Rudolf Jaenisch is a Professor of Biology at MIT and a founding member of the Whitehead Institute for Biomedical Research. He is a pioneer of transgenic science, in which an animal’s genetic makeup is altered. Jaenisch has focused on creating genetically modified mice to study cancer, epigenetic reprogramming and neurological diseases.
Hemangioblasts are the multipotent precursor cells that can differentiate into both hematopoietic and endothelial cells. In the mouse embryo, the emergence of blood islands in the yolk sac at embryonic day 7 marks the onset of hematopoiesis. From these blood islands, the hematopoietic cells and vasculature are formed shortly after. Hemangioblasts are the progenitors that form the blood islands. To date, the hemangioblast has been identified in human, mouse and zebrafish embryos.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka and Kazutoshi Takahashi in Kyoto, Japan, who together showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."
Astellas Institute for Regenerative Medicine is a subsidiary of Astellas Pharma located in Marlborough, Massachusetts, US, developing stem cell therapies with a focus on diseases that cause blindness. It was formed in 1994 as a company named Advanced Cell Technology, Incorporated (ACT), which was renamed to Ocata Therapeutics in November 2014. In February 2016 Ocata was acquired by Astellas for $379 million USD.

Shinya Yamanaka is a Japanese stem cell researcher and a Nobel Prize laureate. He is a professor and the director emeritus of Center for iPS Cell Research and Application, Kyoto University; as a senior investigator at the UCSF-affiliated Gladstone Institutes in San Francisco, California; and as a professor of anatomy at University of California, San Francisco (UCSF). Yamanaka is also a past president of the International Society for Stem Cell Research (ISSCR).
Embryomics is the identification, characterization and study of the diverse cell types which arise during embryogenesis, especially as this relates to the location and developmental history of cells in the embryo. Cell type may be determined according to several criteria: location in the developing embryo, gene expression as indicated by protein and nucleic acid markers and surface antigens, and also position on the embryogenic tree.
Forkhead box D3 also known as FOXD3 is a forkhead protein that in humans is encoded by the FOXD3 gene.
Stem cell laws are the law rules, and policy governance concerning the sources, research, and uses in treatment of stem cells in humans. These laws have been the source of much controversy and vary significantly by country. In the European Union, stem cell research using the human embryo is permitted in Sweden, Spain, Finland, Belgium, Greece, Britain, Denmark and the Netherlands; however, it is illegal in Germany, Austria, Ireland, Italy, and Portugal. The issue has similarly divided the United States, with several states enforcing a complete ban and others giving support. Elsewhere, Japan, India, Iran, Israel, South Korea, China, and Australia are supportive. However, New Zealand, most of Africa, and most of South America are restrictive.
Stem cell laws and policy in the United States have had a complicated legal and political history.

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
Induced stem cells (iSC) are stem cells derived from somatic, reproductive, pluripotent or other cell types by deliberate epigenetic reprogramming. They are classified as either totipotent (iTC), pluripotent (iPSC) or progenitor or unipotent – (iUSC) according to their developmental potential and degree of dedifferentiation. Progenitors are obtained by so-called direct reprogramming or directed differentiation and are also called induced somatic stem cells.
Directed differentiation is a bioengineering methodology at the interface of stem cell biology, developmental biology and tissue engineering. It is essentially harnessing the potential of stem cells by constraining their differentiation in vitro toward a specific cell type or tissue of interest. Stem cells are by definition pluripotent, able to differentiate into several cell types such as neurons, cardiomyocytes, hepatocytes, etc. Efficient directed differentiation requires a detailed understanding of the lineage and cell fate decision, often provided by developmental biology.
Nissim Benvenisty is Professor of Genetics, the Herbert Cohn Chair in Cancer Research and the Director of “The Azrieli Center for Stem Cells and Genetic Research” at the Alexander Silberman Institute of Life Sciences, Hebrew University.












