Related Research Articles

Datura is a genus of nine species of highly poisonous, vespertine-flowering plants belonging to the nightshade family (Solanaceae). They are commonly known as thornapples or jimsonweeds, but are also known as devil's trumpets. Other English common names include moonflower, devil's weed, and hell's bells. All species of Datura are extremely poisonous and psychoactive, especially their seeds and flowers, which can cause respiratory depression, arrhythmias, fever, delirium, hallucinations, anticholinergic syndrome, psychosis, and death if taken internally.

Strychnine is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eyes or mouth, causes poisoning which results in muscular convulsions and eventually death through asphyxia. While it is no longer used medicinally, it was used historically in small doses to strengthen muscle contractions, such as a heart and bowel stimulant and performance-enhancing drug. The most common source is from the seeds of the Strychnos nux-vomica tree.

Syrup of ipecac, or simply ipecac, is a drug that was once widely used as an expectorant and a rapid-acting emetic. It is obtained from the dried rhizome and roots of the ipecacuanha plant, from which it derives its name. It is no longer regularly used in medicine.

Solanine is a glycoalkaloid poison found in species of the nightshade family within the genus Solanum, such as the potato, the tomato, and the eggplant. It can occur naturally in any part of the plant, including the leaves, fruit, and tubers. Solanine has pesticidal properties, and it is one of the plant's natural defenses. Solanine was first isolated in 1820 from the berries of the European black nightshade, after which it was named. It belongs to the chemical family of saponins.

Nicotine poisoning describes the symptoms of the toxic effects of nicotine following ingestion, inhalation, or skin contact. Nicotine poisoning can potentially be deadly, though serious or fatal overdoses are rare. Historically, most cases of nicotine poisoning have been the result of use of nicotine as an insecticide. More recent cases of poisoning typically appear to be in the form of Green Tobacco Sickness, or due to unintended ingestion of tobacco or tobacco products or consumption of nicotine-containing plants.

Theobromine poisoning, also informally called chocolate poisoning or cocoa poisoning, is an overdosage reaction to the xanthine alkaloid theobromine, found in chocolate, tea, cola beverages, and some other foods.

Cyclopamine (11-deoxojervine) is a naturally occurring steroidal alkaloid. It is a teratogenic component of corn lily, which when consumed during gestation has been demonstrated to induce birth defects, including the development of a single eye (cyclopia) in offspring. The molecule was named after this effect, which was originally observed by Idaho lamb farmers in 1957 after their herds gave birth to cycloptic lambs. It then took more than a decade to identify corn lily as the culprit. Later work suggested that differing rain patterns had changed grazing behaviours, which led to a greater quantity of corn lily to be ingested by pregnant sheep. Cyclopamine interrupts the sonic hedgehog signalling pathway, instrumental in early development, ultimately causing birth defects.

Veratridine is a steroidal alkaloid found in plants of the lily family, specifically the genera Veratrum and Schoenocaulon. Upon absorption through the skin or mucous membranes, it acts as a neurotoxin by binding to and preventing the inactivation of voltage-gated sodium ion channels in heart, nerve, and skeletal muscle cell membranes. Veratridine increases nerve excitability and intracellular Ca2+ concentrations.

Veratrum is a genus of flowering plants in the family Melanthiaceae. It occurs in damp habitats across much of temperate and subarctic Europe, Asia, and North America.

Pyrrolizidine alkaloids (PAs), sometimes referred to as necine bases, are a group of naturally occurring alkaloids based on the structure of pyrrolizidine. Their use dates back centuries and is intertwined with the discovery, understanding, and eventual recognition of their toxicity on humans and animals.

Veratrum album, the false helleborine, white hellebore, European white hellebore, or white veratrum is a poisonous plant in the family Melanthiaceae. It is native to Europe and parts of western Asia.

Veratrum nigrum, the black false hellebore, is a widespread Eurasian species of perennial flowering plant in the family Melanthiaceae. Despite its common name, V. nigrum is not closely related to the true hellebores, nor does it resemble them.

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to yohimbine, mitragynine and more distantly Voacangine, all of which are alkaloid plant products with pharmacological properties.

Conium maculatum, known as hemlock, or poison hemlock is a highly poisonous flowering plant in the carrot family Apiaceae, native to Europe and North Africa. It is herbaceous without woody parts and has a biennial lifecycle. A hardy plant capable of living in a variety of environments, hemlock is widely naturalised in locations outside its native range, such as parts of Australia, West Asia, and North and South America, to which it has been introduced. It is capable of spreading and thereby becoming an invasive weed.

Senecionine is a toxic pyrrolizidine alkaloid isolated from various botanical sources. It takes its name from the Senecio genus and is produced by many different plants in that genus, including Jacobaea vulgaris. It has also been isolated from several other plants, including Brachyglottis repanda, Emilia, Erechtites hieraciifolius, Petasites, Syneilesis, Crotalaria, Caltha leptosepala, and Castilleja.

Pipermethystine is a toxic alkaloid present in the aerial (aboveground) portions of the kava plant. It is not a kavalactone, containing no lactone structure. Correctly prepared kava root products will contain almost no pipermethystine.

Riddelliine is a chemical compound classified as a pyrrolizidine alkaloid. It was first isolated from Senecio riddellii and is also found in a variety of plants including Jacobaea vulgaris, Senecio vulgaris, and others plants in the genus Senecio.

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..

Taxine alkaloids, which are often named under the collective title of taxines, are the toxic chemicals that can be isolated from the yew tree. The amount of taxine alkaloids depends on the species of yew, with Taxus baccata and Taxus cuspidata containing the most. The major taxine alkaloids are taxine A and taxine B although there are at least 10 different alkaloids. Until 1956, it was believed that all the taxine alkaloids were one single compound named taxine.
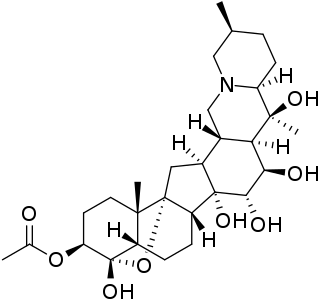
Zygacine is a steroidal alkaloid of the genera Toxicoscordion, Zigadenus, Stenanthium and Anticlea of the family Melanthiaceae. These plants are commonly known and generally referred to as death camas. Death camas is prevalent throughout North America and is frequently the source of poisoning for outdoor enthusiasts and livestock due to its resemblance to other edible plants such as the wild onion. Despite this resemblance, the death camas plant lacks the distinct onion odor and is bitter to taste.
References
- ↑ Carlier P, Efthymiou ML, Garnier R, Hoffelt J, Fournier E (1983). "Poisoning with Veratrum-containing sneezing powders". Human Toxicology. 2 (2): 321–325. doi:10.1177/096032718300200224. PMID 6862477. S2CID 44786035.
- ↑ Fogh A, Kulling P, Wickstrom E (1983). "Veratrum alkaloids in sneezing-powder a potential danger". Journal of Toxicology. Clinical Toxicology. 20 (2): 175–179. doi:10.3109/15563658308990062. PMID 6887310.