Related Research Articles

Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.
Fluoride is an inorganic, monatomic anion of fluorine, with the chemical formula F−
, whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions. It is also used in metallurgy and in medical imaging.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all elements and fluorine has the highest electronegativity of all known elements.
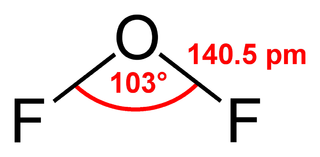
Oxygen difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a "bent" molecular geometry. It is strong oxidizer and has attracted attention in rocketry for this reason. With a boiling point of -144.75 °C, OF2 is the most volatile (isolable) triatomic compound.

Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ammonium hydrogen fluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 C.F.R. 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Zirconium(IV) fluoride (ZrF4) is an inorganic chemical compound. It is a component of ZBLAN fluoride glass. It is insoluble in water. It is the main component of fluorozirconate glasses.

Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin stannum, 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Sodium fluorosilicate is a compound with the chemical formula Na2[SiF6].
Zendium is a brand of toothpaste marketed by Unilever in Austria, Belgium, Croatia, Czech Republic, France, Germany, Hungary, Italy, the Middle East, the Netherlands, Scandinavia, Slovakia and Switzerland.

The bismuth-phosphate process was used to extract plutonium from irradiated uranium taken from nuclear reactors. It was developed during World War II by Stanley G. Thompson, a chemist working for the Manhattan Project at the University of California, Berkeley. This process was used to produce plutonium at the Hanford Site. Plutonium was used in the atomic bomb that was used in the atomic bombing of Nagasaki in August 1945. The process was superseded in the 1950s by the REDOX and PUREX processes.

Amine fluorides are dental drugs.
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation and bifluoride anion. It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.
Sodium salts are salts composed of a sodium cation and the conjugate base anion of some inorganic or organic acids. They can be formed by the neutralization of such acids with sodium hydroxide.