Related Research Articles
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.
Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.

Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.

Sulfites or sulphites are compounds that contain the sulfite ion, SO2−
3. The sulfite ion is the conjugate base of bisulfite. Although its acid is elusive, its salts are widely used.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.
Ammonium lauryl sulfate (ALS) is the common name for ammonium dodecyl sulfate (CH3(CH2)10CH2OSO3NH4). The anion consists of a nonpolar hydrocarbon chain and a polar sulfate end group. The combination of nonpolar and polar groups confers surfactant properties to the anion: it facilitates dissolution of both polar and non-polar materials. This salt is classified as a sulfate ester. It is primarily used in shampoos and body-wash as a foaming agent. Lauryl sulfates are very high-foam surfactants that disrupt the surface tension of water in part by forming micelles at the surface-air interface.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Barium nitrate is the inorganic compound with the chemical formula Ba(NO3)2. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.
Dodecanol, or lauryl alcohol, is an organic compound produced industrially from palm kernel oil or coconut oil. It is a fatty alcohol. Sulfate esters of lauryl alcohol, especially sodium lauryl sulfate, are very widely used as surfactants. Sodium lauryl sulfate, ammonium lauryl sulfate, and sodium laureth sulfate are all used in shampoos. Lauryl alcohol is tasteless and colorless with a floral odor.
A foaming agent is a material such as a surfactant or a blowing agent that facilitates the formation of foam. A surfactant, when present in small amounts, reduces surface tension of a liquid or increases its colloidal stability by inhibiting coalescence of bubbles. A blowing agent is a gas that forms the gaseous part of the foam.

Thallium(I) sulfate (Tl2SO4) or thallous sulfate is the sulfate salt of thallium in the common +1 oxidation state, as indicated by the Roman numeral I. It is often referred to as simply thallium sulfate.

Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly.
Sugar soap, as typically found in Commonwealth countries, is a cleaning material of variable composition sold for use on surfaces affected by greasy or tarry deposits which are not easily removed with routine domestic cleaning materials. Its name arises from the fact that, when in dry powder form, it resembles table sugar.

A chemistry set is an educational toy allowing the user to perform simple chemistry experiments.

Prasterone sulfate, also known as dehydroepiandrosterone sulfate (DHEA-S), is a naturally occurring androstane steroid which is marketed and used in Japan and other countries as a labor inducer in the treatment of insufficient cervical ripening and dilation during childbirth. It is the C3β sulfate ester of prasterone, and is known to act as a prohormone of DHEA and by extension of androgens and estrogens, although it also has its own activity as a neurosteroid. Prasterone sulfate is used medically as the sodium salt via injection and is referred to by the name sodium prasterone sulfate (JAN).
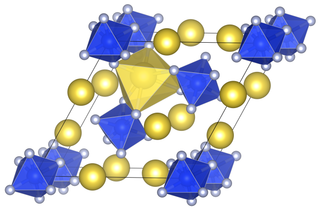
Sodium fluorosilicate is a compound with the chemical formula Na2[SiF6]. Unlike other sodium salts, it has a low solubility in water.
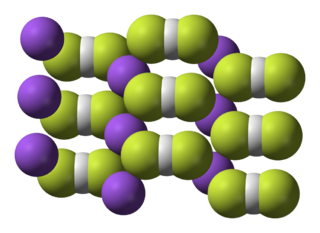
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation and bifluoride anion. It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.
References
- 1 2 3 Mellor, J. W. (1961). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. new impr. London: Longmans. pp. 660–672.
- ↑ "Sodium sulfate". NIST . Retrieved 2007-06-14.
- ↑ "Spectral Database for Organic Compounds". Advanced Industrial Science and Technology. Archived from the original (Queriable database) on 5 May 2006. Retrieved 9 June 2007.