
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) that resembles a soccer ball (football), made of twenty hexagons and twelve pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.

Raman spectroscopy ; named after Indian physicist Sir C. V. Raman) is a spectroscopic technique used to observe vibrational, rotational, and other low-frequency modes in a system. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Fluorescein is a manufactured organic compound and dye. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

β-Carotene is an organic, strongly colored red-orange pigment abundant in plants and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 carbons. Among the carotenes, β-carotene is distinguished by having beta-rings at both ends of the molecule. β-Carotene is biosynthesized from geranylgeranyl pyrophosphate.

Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C
16H
10. This colorless solid is the smallest peri-fused PAH. Pyrene forms during incomplete combustion of organic compounds.

Two-photon excitation microscopy is a fluorescence imaging technique that allows imaging of living tissue up to about one millimeter in depth. It differs from traditional fluorescence microscopy, in which the excitation wavelength is shorter than the emission wavelength, as the wavelengths of the two exciting photons are longer than the wavelength of the resulting emitted light. Two-photon excitation microscopy typically uses near-infrared excitation light which can also excite fluorescent dyes. However, for each excitation, two photons of infrared light are absorbed. Using infrared light minimizes scattering in the tissue. Due to the multiphoton absorption, the background signal is strongly suppressed. Both effects lead to an increased penetration depth for these microscopes. Two-photon excitation can be a superior alternative to confocal microscopy due to its deeper tissue penetration, efficient light detection, and reduced photobleaching.
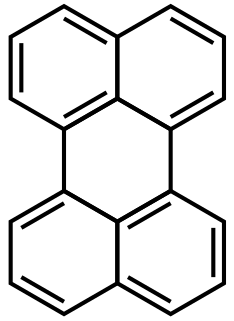
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes.

Texas Red or sulforhodamine 101 acid chloride is a red fluorescent dye, used in histology for staining cell specimens, for sorting cells with fluorescent-activated cell sorting machines, in fluorescence microscopy applications, and in immunohistochemistry. Texas Red fluoresces at about 615 nm, and the peak of its absorption spectrum is at 589 nm. The powder is dark purple. Solutions can be excited by a dye laser tuned to 595-605 nm, or less efficiently a krypton laser at 567 nm. The absorption extinction coefficient at 596 nm is about 85,000 M−1cm−1.

Quenching refers to any process which decreases the fluorescence intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on pressure and temperature. Molecular oxygen, iodide ions and acrylamide are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence. Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence.

Solvatochromism is the term used to describe the phenomenon that is observed when the colour due to a solute is different when that solute is dissolved in different solvents.

Fluorene, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar. It is insoluble in water and soluble in many organic solvents.

A molecular logic gate is a molecule that performs a logical operation based on one or more physical or chemical inputs and a single output. The field has advanced from simple logic systems based on a single chemical or physical input to molecules capable of combinatorial and sequential operations such as arithmetic operations i.e. moleculators and memory storage algorithms.
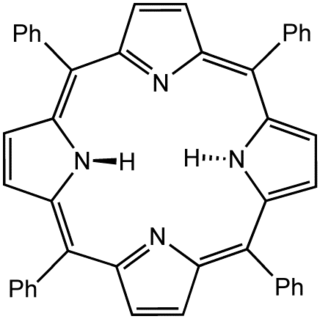
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.
A J-aggregate is a type of dye with an absorption band that shifts to a longer wavelength of increasing sharpness when it aggregates under the influence of a solvent or additive or concentration as a result of supramolecular self-organisation. The dye can be characterized further by a small Stokes shift with a narrow band. The J in J-aggregate refers to E.E. Jelley who discovered the phenomenon in 1936. The dye is also called a Scheibe aggregate after G. Scheibe who also independently published on this topic in 1937.

Carbon quantum dots are small carbon nanoparticles with some form of surface passivation.
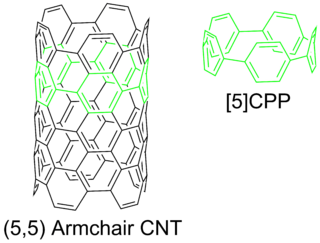
Cycloparaphenylenes (CPPs) are cylindrical molecules made of para-linked benzene rings in a hoop-like structure. For this reason they are sometimes referred to as carbon nanohoops, especially because they can be viewed as a fragment of an armchair carbon nanotube (CNT). CPPs represent a challenging target to synthetic chemists due to the ring strain incurred from forcing benzene rings out of planarity.
























