Related Research Articles

Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.

In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series or "positive feedback loop" of these reactions. The specific nuclear reaction may be the fission of heavy isotopes. A nuclear chain reaction releases several million times more energy per reaction than any chemical reaction.
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal chain reaction can only be achieved with fissile material. The predominant neutron energy in a system may be typified by either slow neutrons or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives.

In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties, density, shape, enrichment, purity, temperature, and surroundings. The concept is important in nuclear weapon design.

A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear power. Neutron source variables include the energy of the neutrons emitted by the source, the rate of neutrons emitted by the source, the size of the source, the cost of owning and maintaining the source, and government regulations related to the source.
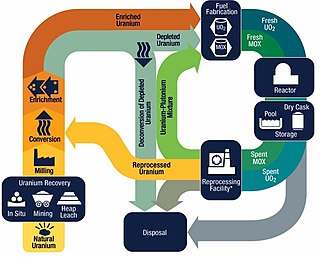
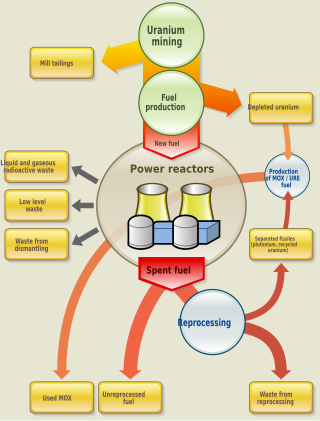
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

A fast-neutron reactor (FNR) or fast-spectrum reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons, as opposed to slow thermal neutrons used in thermal-neutron reactors. Such a fast reactor needs no neutron moderator, but requires fuel that is relatively rich in fissile material when compared to that required for a thermal-neutron reactor. Around 20 land based fast reactors have been built, accumulating over 400 reactor years of operation globally. The largest was the Superphénix sodium cooled fast reactor in France that was designed to deliver 1,242 MWe. Fast reactors have been studied since the 1950s, as they provide certain advantages over the existing fleet of water-cooled and water-moderated reactors. These are:
A subcritical reactor is a nuclear fission reactor concept that produces fission without achieving criticality. Instead of sustaining a chain reaction, a subcritical reactor uses additional neutrons from an outside source. There are two general classes of such devices. One uses neutrons provided by a nuclear fusion machine, a concept known as a fusion–fission hybrid. The other uses neutrons created through spallation of heavy nuclei by charged particles such as protons accelerated by a particle accelerator, a concept known as an accelerator-driven system (ADS) or accelerator-driven sub-critical reactor.

The integral fast reactor (IFR), originally the advancedliquid-metal reactor (ALMR), is a design for a nuclear reactor using fast neutrons and no neutron moderator. IFRs can breed more fuel and are distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other nuclear devices to generate energy.
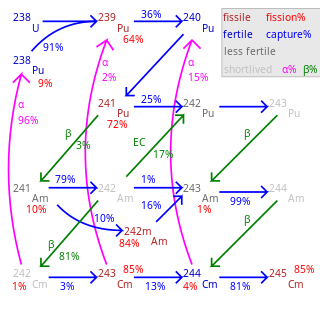
Fertile material is a material that, although not fissile itself, can be converted into a fissile material by neutron absorption.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty-two plutonium radioisotopes have been characterized. The most stable are 244Pu with a half-life of 80.8 million years; 242Pu with a half-life of 373,300 years; and 239Pu with a half-life of 24,110 years; and 240Pu with a half-life of 6,560 years. This element also has eight meta states; all have half-lives of less than one second.
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy.
In applications such as nuclear reactors, a neutron poison is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant.
Plutonium-240 is an isotope of plutonium formed when plutonium-239 captures a neutron. The detection of its spontaneous fission led to its discovery in 1944 at Los Alamos and had important consequences for the Manhattan Project.
Uranium-236 is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.

The liquid fluoride thorium reactor is a type of molten salt reactor. LFTRs use the thorium fuel cycle with a fluoride-based molten (liquid) salt for fuel. In a typical design, the liquid is pumped between a critical core and an external heat exchanger where the heat is transferred to a nonradioactive secondary salt. The secondary salt then transfers its heat to a steam turbine or closed-cycle gas turbine.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
References
- ↑ Atomic Energy of Canada (1997). Canada enters the nuclear age: a technical history of Atomic Energy of Canada Limited. McGill-Queen's Press - MQUP. p. 224. ISBN 0-7735-1601-8.
- 1 2 U.S. patent 4,208,247 Neutron source
- ↑ "Microsoft Word - lecture25.doc" (PDF). Archived from the original (PDF) on June 29, 2011. Retrieved 2010-03-28.
- ↑ Ken Kok (2009). Nuclear Engineering Handbook. CRC Press. p. 27. ISBN 978-1-4200-5390-6.
- 1 2 Integrated Publishing. "Neutron Sources Summary". Tpub.com. Retrieved 2010-03-28.
- ↑ Karl-Heinz Neeb (1997). The radiochemistry of nuclear power plants with light water reactors. Walter de Gruyter. p. 147. ISBN 3-11-013242-7.
- ↑ "Memorandum from Raymond L. Murray to Dr. Clifford K. Beck". Lib.ncsu.edu. Retrieved 2010-03-28.