The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen, carbon dioxide, methane, and water vapour —from coal and water, air and/or oxygen.

Flue-gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide from exhaust flue gases of fossil-fuel power plants, and from the emissions of other sulfur oxide emitting processes such as waste incineration, petroleum refineries, cement and lime kilns.
Sour gas is natural gas or any other gas containing significant amounts of hydrogen sulfide (H2S).

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.

Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from gases. It is a common unit process used in refineries, and is also used in petrochemical plants, natural gas processing plants and other industries.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases, as from a fireplace, oven, furnace, boiler or steam generator. It often refers to the exhaust gas of combustion at power plants. Technology is available to remove pollutants from flue gas at power plants.

Synthetic fuel or synfuel is a liquid fuel, or sometimes gaseous fuel, obtained from syngas, a mixture of carbon monoxide and hydrogen, in which the syngas was derived from gasification of solid feedstocks such as coal or biomass or by reforming of natural gas.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
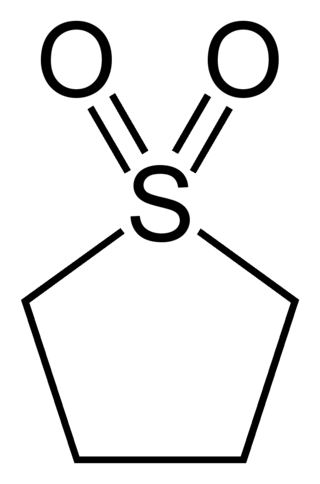
Sulfolane is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent for extractive distillation and chemical reactions. Sulfolane was originally developed by the Shell Oil Company in the 1960s as a solvent to purify butadiene. Sulfolane is a polar aprotic solvent, and it is miscible with water.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency, compared to conventional pulverized coal combustion.
Merox is an acronym for mercaptan oxidation. It is a proprietary catalytic chemical process developed by UOP used in oil refineries and natural gas processing plants to remove mercaptans from LPG, propane, butanes, light naphthas, kerosene, and jet fuel by converting them to liquid hydrocarbon disulfides.

Natural-gas processing is a range of industrial processes designed to purify raw natural gas by removing contaminants such as solids, water, carbon dioxide (CO2), hydrogen sulfide (H2S), mercury and higher molecular mass hydrocarbons (condensate) to produce pipeline quality dry natural gas for pipeline distribution and final use. Some of the substances which contaminate natural gas have economic value and are further processed or sold. Hydrocarbons that are liquid at ambient conditions: temperature and pressure (i.e., pentane and heavier) are called natural-gas condensate (sometimes also called natural gasoline or simply condensate).

Natural gas has been used almost as long as crude oil in Canada, but its commercial development was not as rapid. This is because of special properties of this energy commodity: it is a gas, and it frequently contains impurities. The technical challenges involved to first process and then pipe it to market are therefore considerable. Furthermore, the costs of pipeline building make the whole enterprise capital intensive, requiring both money and engineering expertise, and large enough markets to make the business profitable.
Black powder is an industry name for the abrasive, reactive particulate contamination present in all gas and hydrocarbon fluid transmission lines. Black powder ranges from light brown to black, and the mineral makeup varies per production field around the world.
The Shell–Paques process, also known by the trade name of Thiopaq O&G, is a gas desulfurization technology for the removal of hydrogen sulfide from natural-, refinery-, synthesis- and biogas. The process was initially named after the Shell Oil and Paques purification companies. After accession of a dedicated joint venture by the founders, Paqell B.V., the trade name for applications in the Oil & Gas industry was changed to "THIOPAQ O&G". It is based on the biocatalytical conversion of sulfide into elemental sulfur. It operates at near-ambient conditions of temperature, about 30-40 °C, and pressure which results in inherent safety. It is an alternative to, for example, the Claus process.
The wet sulfuric acid process (WSA process) is a gas desulfurization process. After Danish company Haldor Topsoe introduced this technology in 1987, it has been recognized as a process for recovering sulfur from various process gases in the form of commercial quality sulfuric acid (H2SO4) with the simultaneous production of high-pressure steam. The WSA process can be applied in all industries where sulfur removal presents an issue.
CrystaSulf is the trade name for a chemical process used for removing hydrogen sulfide (H2S) from natural gas, synthesis gas and other gas streams in refineries and chemical plants. CrystaSulf uses a modified liquid-phase Claus reaction to convert the hydrogen sulfide (H2S) into elemental sulfur which is then removed from the process by filtration. CrystaSulf is used in the energy industry as a mid-range process to handle sulfur amounts between 0.1 and 20 tons per day. Below 0.1 tons of sulfur per day is typically managed by H2S Scavengers and applications above 20 tons per day are typically treated with the Amine – Claus process.
The SNOX process is a process which removes sulfur dioxide, nitrogen oxides and particulates from flue gases. The sulfur is recovered as concentrated sulfuric acid and the nitrogen oxides are reduced to free nitrogen. The process is based on the well-known wet sulfuric acid process (WSA), a process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4).









